-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![cGMP ssDNA And dsDNA Services Are Here! cGMP ssDNA And dsDNA Services Are Here!]()
cGMP HDR Templates
Clinical grade ssDNA/dsDNA and circular dsDNA for therapeutic development
Home » CRISPR Services » Homology-Directed Repair (HDR) Knock-in Templates » cGMP ssDNA and dsDNA Services
Overview
As non-viral CRISPR gene insertion methods gain traction in advanced cell and gene therapy programs, GenScript offers industry-leading cGMP-compliant HDR template solutions to support your journey from research to clinical trials. Our clinical-grade GenExact™ single-stranded DNA (ssDNA), GenWand™ closed-end linear double-stranded DNA (dsDNA), and GenCircle™ circular dsDNA templates are engineered to meet the rigorous demands of CRISPR-based therapy development and IND-enabling studies.
Manufactured in state-of-the-art ISO Class 7 cleanrooms with ISO Class 5 isolators for aseptic processing, our cGMP facilities operate under a robust Quality Management System aligned with ICH Q7 guidelines for clinical use. GenScript’s cGMP HDR templates empower faster, more efficient progression from R&D to regulatory submission and clinical manufacturing.
Why Choose GenScript for Clinical-Grade Non-Viral Gene and Cell Therapy Reagents?
1/Non-Viral HDR Template Leader
Innovator and leader in long and complex ssDNA and dsDNA HDR template synthesis for non-viral CRISPR applications.
2/IND Filing Support
Full regulatory documentation provided, including CoAs and quality certificates, to support IND submissions and clinical trial readiness.
3/Faster Cell Therapy Development
Non-viral HDR templates enable safer, faster CRISPR cell engineering by eliminating reliance on traditional viral vectors.
4/From Discovery to Clinical Trials
We offer RUO, INDEdit, and cGMP reagents to support every stage of CRISPR therapy development.
5/Custom-Built to Your Needs
Fully customizable format, sequence, and scale:
- GenExact™ ssDNA: up to 6,000 nt, 100 mg/batch
- GenWand™ linear dsDNA: up to 10 kb, gram scale
- GenCircle™ circular dsDNA: up to 20 kb, gram scale
6/High Quality, Full cGMP Compliance
Manufactured under ICH Q7-compliant cGMP conditions with validated QC for purity, sterility, stability, and more.
7/Our Track Record
- 69+ cGMP HDR Template batches successfully delivered
- 7 global IND approvals
- OmniGuide RNA
- HDR Templates
- gRNA + HDR Design Tools
- Plasmid gRNA
- gRNA Libraries
- Cell Engineering
- Microbial Editing
- Cas9 Nucleases
GenScript’s cGMP Capabilities for CRISPR Guide RNA and HDR Template Manufacturing
Specifications
cGMP INDEdit RUO Production Site cGMP site cGMP site RUO site Application/ Purpose Phase I, II, III
& commercialPreclinical research to
IND enabling studiesDiscovery & development Quality Management
System (QMS)cGMP-compliant cGMP-compliant
(simplified)ISO 9001 Batch Record √ √ x Analytical Method
Qualification/ ValidationValidated Not required
(Platform Standards)Not required QC Tests Basic + Advanced + Premium Basic + Advanced Basic QA Release Yes Yes Yes Delivered Documentations 1.Cert. of Analysis (CoA)
2.TSE/BSE Statement
3.Cert. of Compliance (CoC) 1.Cert. of Analysis (CoA)
2.TSE/BSE Statement 1.Cert. of Analysis (CoA)
2.TSE/BSE StatementNote: *Engineering Run service also available upon request
GenScript’s cGMP Capabilities
Production Environment
- cGMP designated manufacturing lines/rooms
- Controlled production environment
- Cleaning process and cleaning records in place
- Qualified sterile filtration and aseptic processing fill/finish process
- Rx-360 Audit Report (JA-3136) available for licensing—streamline auditing and ensure regulatory confidence, without the need for an on-site audit.
Process Development
- Process development and optimization
- Analytical test method development and optimization
- CQA/CPP Confirmation
Quality Assurance & Documentation
- Material management and supplier management in compliance with cGMP regulations
- Phase appropriate cGMP deployment based on stage of clinical trial and process understanding
- Basic Quality Management Systems in place (Training, Deviations, CAPA,...)
- Manufacturing Summary Report, TSE/BSE Statement
- Batch Records based on Master Batch Record and Change Control system
Quality Control
- Identity, purity, sterility, etc. clearly defined
- Scientific sound QC test methods
Stability Test
- Stability test under various solutions and storage conditions
- Prepare/initiate long term stability studies
Your reliable partner from bench to clinic
GenScript offers the manufacturing expertise, capacity, and validated platform processes to accelerate your non-viral gene and cell engineering from early-phase research to the clinic. Our complete suite of phase-specific CRISPR gene editing solutions provides the flexibility to support your project at any stage.
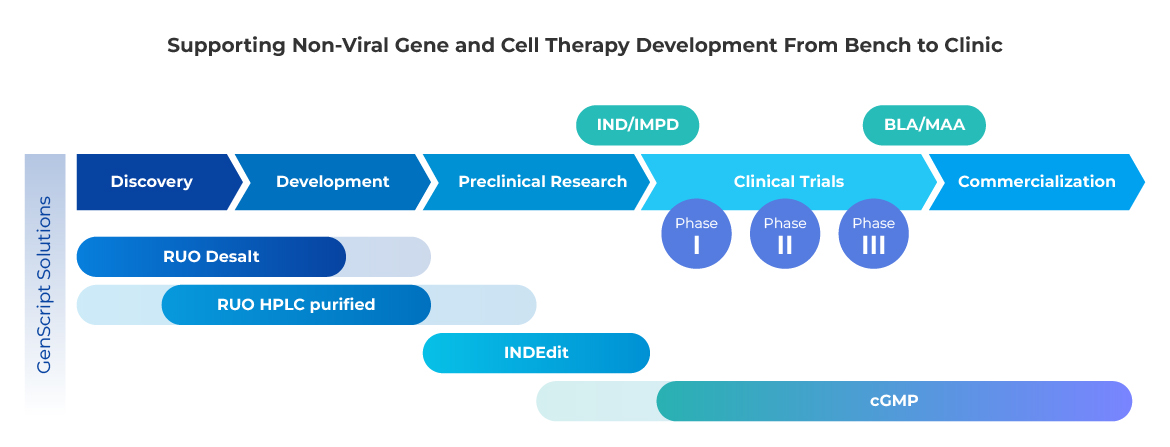
Partner with GenScript to reliably source CRISPR gene editing materials in the quantity and purity you need, and with the documentation required for successful IND submission and clinical trials.
Resources

White Paper: From Concept to Clinic: Navigating the Regulatory Path of CRISPR-based Therapeutics
Free Download
White Paper: Ensuring Quality and Compliance: CMC Strategy for CRISPR-based Therapeutics
Free Download
White Paper: Ensuring Translational Success: Preclinical Study Design for CRISPR-based Therapeutics
Free DownloadStrategic Cooperation

Brian Shy, MD, PhD
Evotec (France) SAS
"We were very happy to partner with Genscript on critical experiments demonstrating high efficiency and yield of CAR knock-in cells at clinical scale. The long ssDNA produced by Genscript exceeded our expectations and helped us clearly demonstrate the potential for future therapeutic applications using these methods.“
Related reading: Cell and Gene Therapy Companies Leverage GenScript’s cGMP sgRNA Capability to Accelerate Speed to Market
BRL Medicine Inc.,
On November 5, 2024, BRL Medicine Inc. announced that its developed "Targeted CD19 Non-Viral PD1 Site-Specific Integration CAR-T Cell Injection" (BRL-203) has officially received Investigational New Drug (IND) approval from China National Medical Products Administration (NMPA) for the clinical treatment of "moderate to severe refractory systemic lupus erythematosus (SLE).“
GenScript provided core materials, GMP sgRNA, and gene knock-in templates (dsDNA) for this approved non-viral CAR-T therapy. This contributes to the development of safer, more efficient, and cost-effective cell therapies based on CRISPR technology and supports the treatment of a more diverse range of diseases.
Ms. Jiang Hua
CEO - Boan Biotech
“GenScript Biotech is a world-leading life science service provider. We fully recognize the services and products that GenScript provides, and expect that our joint efforts will make Boan Biotech’s cell therapy products benefiting patients in clinical practice as soon as possible.”
Related reading: GenScript and Boan Biotech reached a strategic partnership on GMP level GenCircleTM dsDNA, accelerating the process of non-viral CGT developmentWant to know more about our cGMP ssDNA and dsDNA services?

 Hi!Ask me about GenScript services and products! I can answer questions or connect you to a live person.
Hi!Ask me about GenScript services and products! I can answer questions or connect you to a live person. -




































