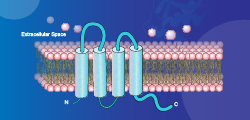
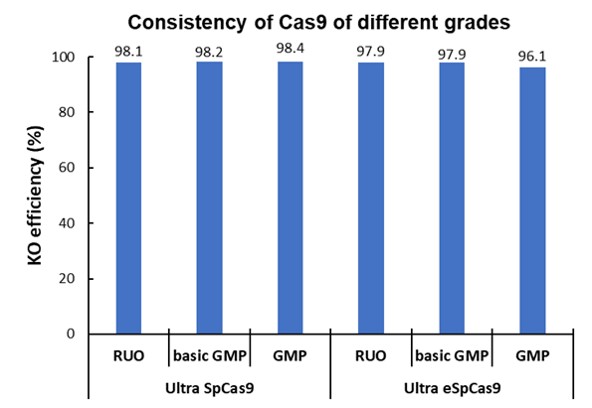
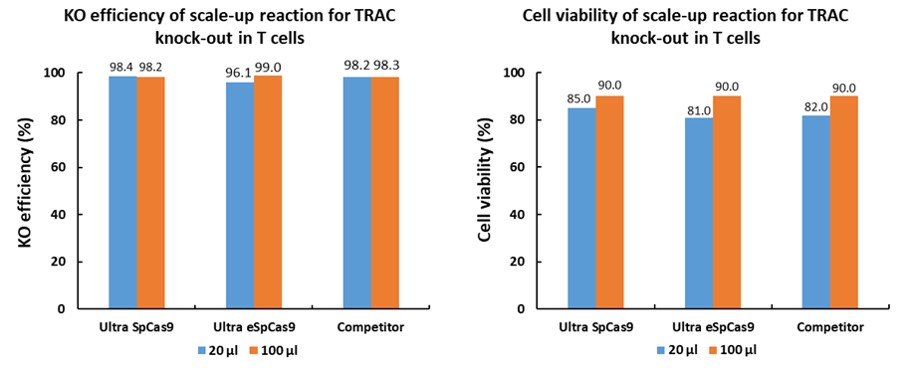
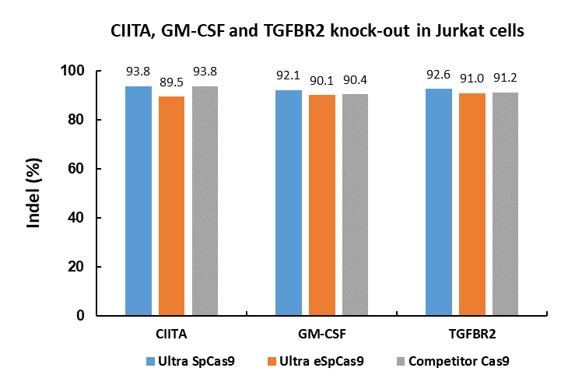
| Test | ||||
|---|---|---|---|---|
| GenCRISPR™ Ultra Cas9 and eSpCas9 Nucleases | ||||
| GMP Grade* | Basic GMP Grade** | RUO Grade | ||
| Facility | GMP Compliant | Non-GMP Compliant | Non-GMP Compliant | |
| cGMP Guidelines | +++ | ++ | + | |
| ISO9001 | / | / | +++ | |
| ISO13485 | +++ | +++ | / | |
| Quality Control | +++ | +++ | + | |
| Documentation | +++ | ++ | + | |
Note:
*GMP Grade is a specific term that GenScript uses to describe the Cas9 nucleases manufactured in GMP-complaint facility and in compliance with guidelines of Current Good Manufacturing Practice (cGMP), ISO 13485 quality management system standards with stringent process controls and complete documentation records. GenScript is capable of providing documents, site audits, and other support to help with the applications of your projects in specific regions.
**Basic GMP Grade is a branding term that GenScript uses to describe reagents manufactured in compliance with ISO 13485 quality management system standards and with more stringent process controls and relatively complete documentation records. GenScript is capable of providing documents, site audit and other support to help with the applications of your projects in specific regions.