Goal: Generate mouse monoclonal antibodies specific to 7-transmembrane protein hCCR9
Immunogen: hCCR9-mRNA-LNP
Screening Material: hCCR9-overexpressing CHO-K1
Challenges: No available purified protein & failed in DNA immunization
Results: Successfully generate 9 hCCR9-positive clones and 7 validated clones (client selected)
After one round of cell fusion
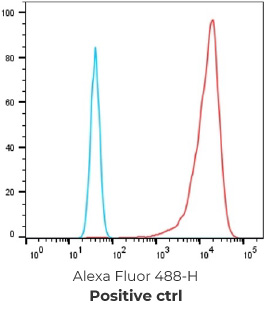
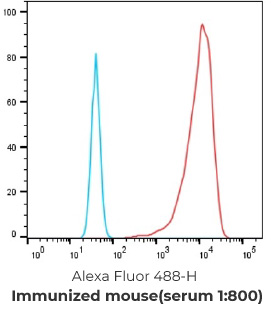
19 FACS positive parental clones
9 FACS positive stable hybridoma cell line & NGS results (with varies IgG1, IgG2a, IgG2b and IgG3 isotypes antibodies identified)
7 validated clones delivered
Isotype identification of 9 hybridoma clones
| Isotype | IgG1, k | IgG2a, k | IgG2b, k | IgG3, k |
|---|---|---|---|---|
| Clone | 58D7, 69D11, 80E9 | 69C5 | 50C11, 50G8, 53B4, 56B3 | 77F5 |
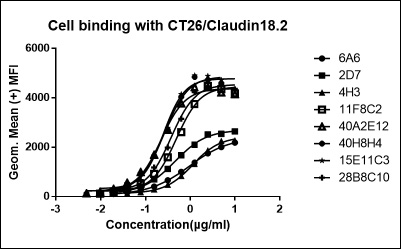
FACS EC50 of 7 recombinant antibody

| 50C11-1 | 50G8-1 | 53B4-1 | 58D7-1 | 69C5-1 | 77F5-1 | 80E9-1 | |
|---|---|---|---|---|---|---|---|
| EC50 | 0.7974 | 2.121 | 0.7245 | 0.1847 | 0.6506 | 0.4246 | 0.266 |
| R square | 0.9997 | 0.9996 | 0.9987 | 0.9922 | 0.9997 | 0.9808 | 0.9899 |
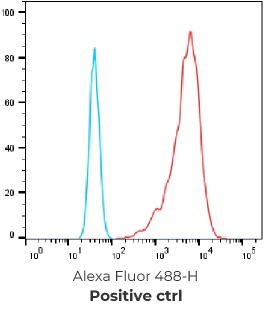
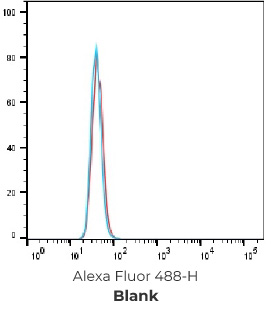
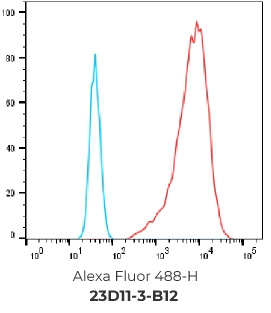
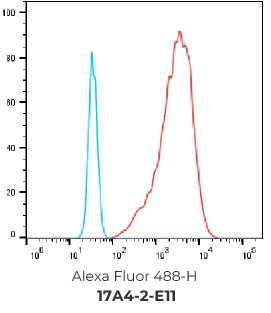
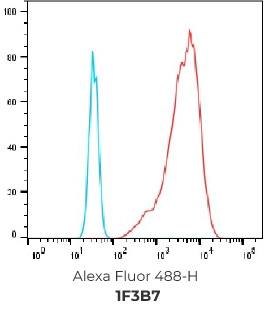
FACS Results of 7 validated hybridoma clones




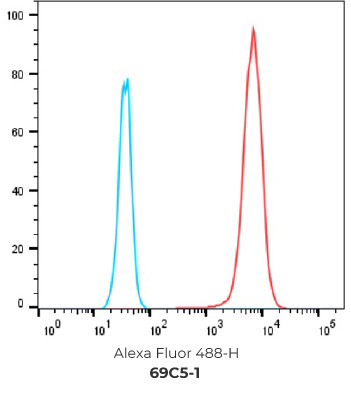

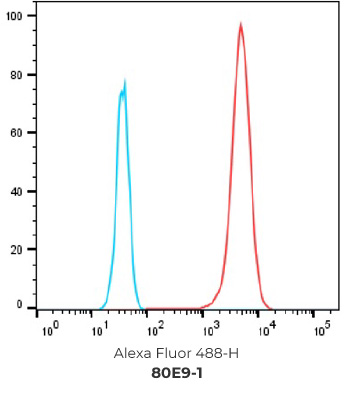
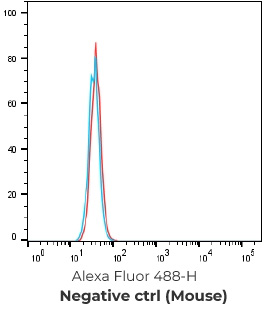
Negative control cells
Positive overexpressed cells