-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![Cell therapy Cell therapy]()
Cell Therapy Solutions
Ready-to-Use, Next-day Shipping
Catalog Products » Cell Therapy Solutions
Cell therapy development is a complex and time-consuming process. GenScript product solutions are designed to streamline and automate your workflow from discovery to commercialization including ready-to-use, high-quality cGMP ancillary materials, a closed cell isolation/depletion system, and comprehensive safety testing.

Expert Support and Service

GMP facilities and Manufacturing excellence

Quality and Regulatory Compliance with FDA and EMA

Seamless Scale-up from pre-IND to commercialization
Workflow
Early DiscoveryPreclinical DevelopmentCMC and ClinicalCAR/TCR Construct Generation

Reagents
Cloning Kits
Plasmid Purification Systems
CAR/TCR
Purification
Automated Protein/Antibody Purification
Protein/Antibody Purification Beads and Resins
More >>One-stop Western Blot Systems
CAR/TCR
Validation
Binding Assays
Cell Processing

Sample Preparation/Thawing
Cell Isolation and Activation
Cell Expansion
Preclinical Validation

Viral Vector Titration
Efficacy Assays
Cell Processing

Sample Preparation/Thawing
Cell Isolation and Activation
Cell Expansion
Analytical and Quality

Viral Vector Titration
Residual Detection
Efficacy Assays
Resources


Cell Isolation and Activation
Free Download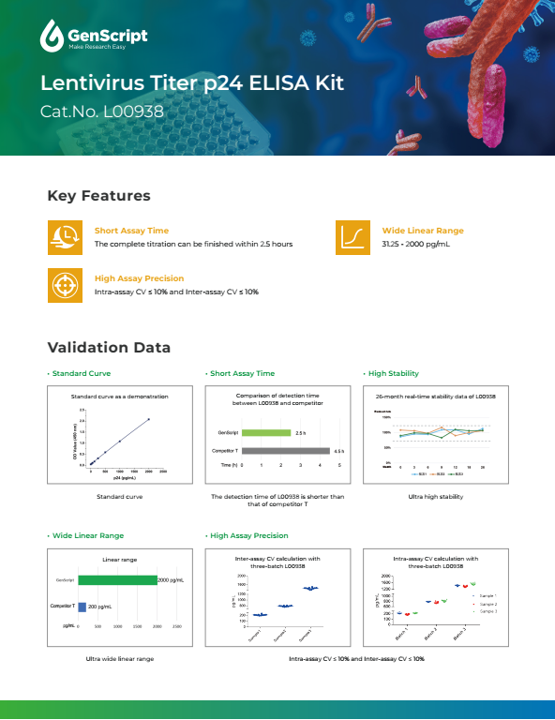
Lentivirus Titer p24 ELISA Kit
Free Download
MonoRab™ Anti-VHH Antibodies
Unique Tools for Detecting Camelid/Humanized Single Domain Antibodies
Free DownloadRelated Products

GMP Grade Cell Isolation and Activation
GMP Grade Cell Isolation and Activation
View more
CytoSinct™ 1000 Cell Isolatoin Instrument
CytoSinct™ 1000 Cell Isolatoin Instrument
View more
Viral Titration QC ELISA Kits
Viral Titration QC ELISA Kits
View more
Residual Detection ELISA Kits
Residual Detection ELISA Kits
View more
Catalog Antibodies
Catalog Antibodies
View more
Catalog Recombinant Proteins
Catalog Recombinant Proteins
View more Hi!Ask me about GenScript services and products! I can answer questions or connect you to a live person.
Hi!Ask me about GenScript services and products! I can answer questions or connect you to a live person. -





































