Your search returns no product results. Please explore our custom peptide synthesis solutions.
-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![Products for ADC Pharmacokinetic Study Products for ADC Pharmacokinetic Study]()
Products for ADC Pharmacokinetic Study
Catalog Products » Antibodies » ADC PK Study Tools
Overview
Antibody-drug conjugates (ADCs) are biopharmaceutical products that consist of a monoclonal antibody conjugated to a small cytotoxic molecule drug (payload) through a chemical linker (Figure 1). They can accurately deliver potent cell-killing payloads to cancer cells by harnessing the highly specific targeting property of monoclonal antibodies, thus, minimizing damage to healthy cells. HER2, CD22, BCMA, and Claudin18.2 are popular target antigens of ADC drugs. MMAE, MMAF, DM1, and DXd are the most commonly used ADC payloads.
Understanding the pharmacokinetics (PK) properties of ADCs in pre-clinical and clinical studies is critical for their successful development. ELISA is a widely used LBA (ligand binding assay) method for ADC PK studies. GenScript provides a comprehensive portfolio of anti-payload antibodies, anti-idiotype antibodies, anti-human IgG Fc antibody, and recombinant target proteins to facilitate your ADC PK studies.
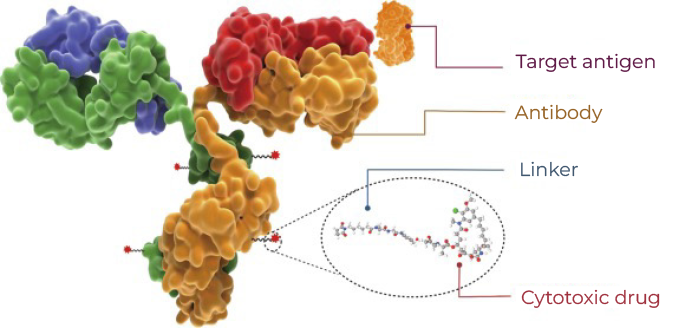
Figure 1. Model structure of ADC.
Retrieved from Fu et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Nature, 2022Highlights
1/High Specificity
- Recognize a particular payload
- No cross-reactivity with other payloads
- Bind to both free and conjugated payloads
2/Perfect Qualification
- Good performance in two LBA methods
- Broad detection range with good linearity and sensitivity
- Ideal for the development of the conjugated and total ADC assays
3/Comprehensive Validation
- Comprehensive validation based on the ICH M10 guidelines
- Valid MRD (minimal required dilution) test with cynomolgus monkey serum
ADC PK Study
Pharmacokinetics is the study and characterization of the time course of drug absorption, distribution, metabolism, and excretion (ADME). Due to the complexity of ADCs that combine an antibody, a cytotoxic drug (also known as payload), and a specialized chemical linker, there are several analytes that need to be measured to characterize ADC PK profiles, including
total antibody, which is the ADC with a drug-antibody ratio (DAR) higher than or equal to zero
drug-conjugated antibody, which is the ADC with a DAR greater than or equal to one
free drugs (payloads) or their metabolites
ELISA immunoassay is a commonly used quantitative analytical method to measure the total and drug-conjugated antibody kinetic profiles. Multiple formats as shown in Figure 2 can be applied to detect target analytes.
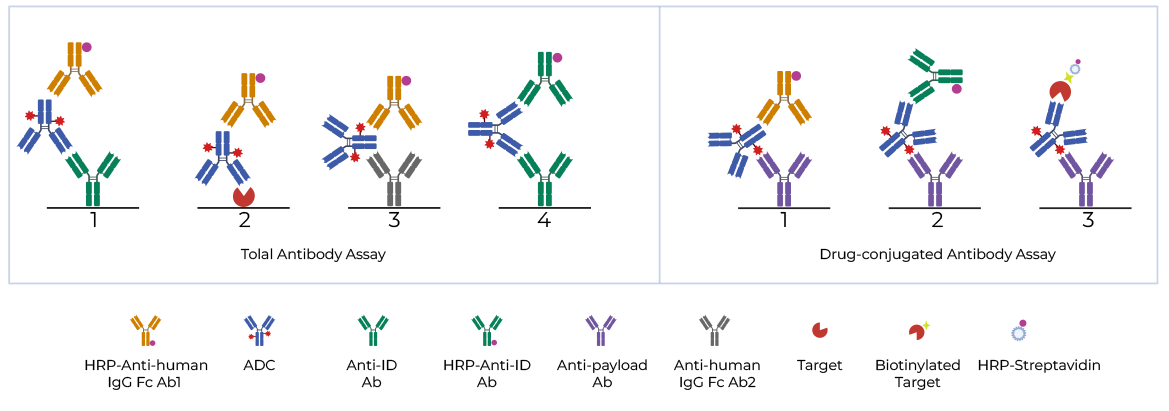
Figure 2. Schematic images of ELISA-based ADC PK study
Items Formats LBA (Capture-Detection) Total Antibody Assay 1 anti-idiotype antibody - anti-human IgG Fc antibody 2 target antigen - anti-human IgG Fc antibody 3 anti-human IgG Fc antibody - anti-human IgG Fc antibody 4 anti-idiotype antibody (A) - anti-idiotype antibody (B) Drug-conjugated antibody Assay 1 anti-payload antibody - anti-human IgG Fc antibody 2 anti-payload antibody - anti-idiotype antibody 3 anti-payload antibody - target antigen Products
GenScript provides anti-payload antibodies, anti-idiotype antibodies, anti-human IgG Fc antibody, and recombinant target proteins for measuring the conjugate and total antibody in ELISA immunoassays.
-
Anti-payload Antibodies
-
Anti-idiotype Antibodies
Anti-Cetuximab/Erbitux (Target: EGF Receptor)
Cat. No. Product Name Quantity Price Order A01938Anti-Cetuximab Antibody (18D5), mAb, Mouse $139.00 40 μg A01939Anti-Cetuximab Antibody (20H1), mAb, Mouse $139.00 40 μg A01991MonoRab™ Anti-Cetuximab Antibody (69H10), mAb, Rabbit $198.00 40 μg A01992MonoRab™ Anti-Cetuximab Antibody (90G3), mAb, Rabbit $198.00 40 μg Anti-Daratumumab/Darzalex (Target: CD38)
Cat. No. Product Name Quantity Price Order A01996Anti-Daratumumab Antibody (11F2), mAb, Mouse $139.00 40 μg A01997Anti-Daratumumab Antibody (6A3), mAb, Mouse $139.00 40 μg Anti-Obinutuzumab/Gazyva (Target: CD20)
Cat. No. Product Name Quantity Price Order A01945Anti-Obinutuzumab Antibody (18H8), mAb, Mouse $139.00 40 μg A01946Anti-Obinutuzumab Antibody (16B7), mAb, Mouse $139.00 40 μg A01966MonoRab™ Anti-Obinutuzumab Antibody (8G12), mAb, Rabbit $198.00 40 μg A01967MonoRab™ Anti-Obinutuzumab Antibody (169F10), mAb, Rabbit $198.00 40 μg Anti-Panitumumab/Vectibix (Target: EGF Receptor)
Cat. No. Product Name Quantity Price Order A02099Anti-Panitumumab Antibody (2E7), mAb, Mouse $139.00 40 μg A02100Anti-Panitumumab Antibody (9G12), mAb, Mouse $139.00 40 μg Anti-Pertuzumab/Perjeta (Target: HER2)
Cat. No. Product Name Quantity Price Order A02150Anti-Pertuzumab Antibody (1A3), mAb, Mouse $139.00 40 μg A02151Anti-Pertuzumab Antibody (11H4), mAb, Mouse $139.00 40 μg Anti-Rituximab/Rituxan (Target: CD20)
Cat. No. Product Name Quantity Price Order A01942MonoRab™ Anti-Rituximab Antibody (137C6), mAb, Rabbit $198.00 40 μg A01943MonoRab™ Anti-Rituximab Antibody (194D7), mAb, Rabbit $198.00 40 μg A01969Anti-Rituximab Antibody (6C1), mAb, Mouse $139.00 40 μg A01970Anti-Rituximab Antibody (17B6), mAb, Mouse $139.00 40 μg Anti-Trastuzumab/Herceptin (Target: HER2)
Cat. No. Product Name Quantity Price Order A02032Anti-Trastuzumab Antibody (11C4), mAb, Mouse $139.00 40 μg A02033Anti-Trastuzumab Antibody (15H2), mAb, Mouse $139.00 40 μg Find More Anti-idiotype Antibodies!
-
Anti-human IgG Fc Antibody
Cat. No. Product Name Quantity Price Order A01854Mouse Anti-Human IgG Fc Antibody (50B4A9)[HRP], mAb $105.00 200 μl -
Recombinant Target Proteins
Cat. No. Product Name Quantity Price Order Z02731BCMA, Human $165.00 Z03194EGFR, His, Human $90.00 Z03504CLDN18.2, His, Human $220.00 Z03642CD30, His, Human $200.00 Z03643Siglec-2/CD22, hFc, Human $220.00 100 μg
Case Study
-
Drug-conjugated Antibody measurement by anti-payload antibody and
anti-idiotype antibody
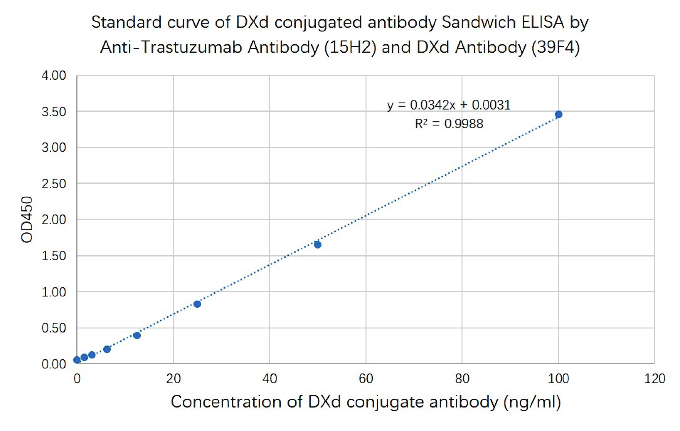
Figure 3. Standard curve of DXd conjugated Trastuzumab Sandwich ELISA by anti-idiotype antibody and DXd Antibody. Based on drug-conjugated antibody assay format 2.
The Sandwich ELISA assay was developed using Anti-Trastuzumab Antibody (15H2), mAb, Mouse (GenScript, A02033) and DXd Antibody (39F4), mAb, Mouse (GenScript, A02217) and as the capture and detection antibodies, respectively.
In this ELISA assay, DXd Antibody (39F4), mAb, Mouse (GenScript, A02217) was labeled with HRP. GenScript can provide customized conjugation service for this product per customer's request.
Capture antibody: Anti-Trastuzumab Antibody (15H2), mAb, Mouse, 2 μg/ml
Detection antibody: DXd Antibody (39F4), mAb, Mouse, 0.22 μg/ml. -
Drug-conjugated Antibody measurement by anti-payload antibody and
anti-Human IgG Fc antibody
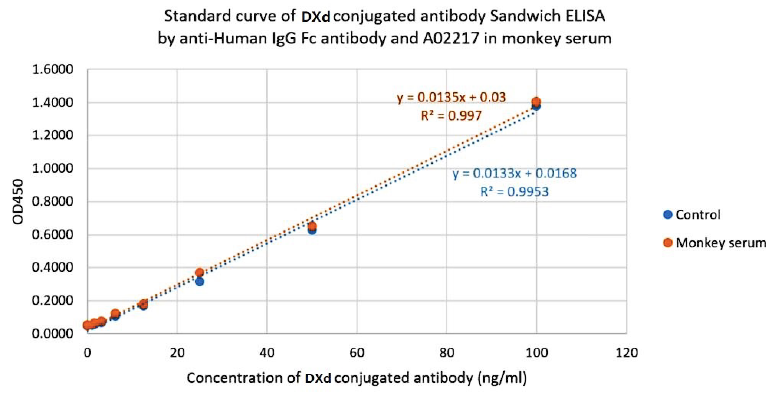
Figure 4. Standard curve of DXd conjugated Trastuzumab Sandwich ELISA by anti-Human IgG Fc antibody and DXd antibody in monkey serum. Based on drug-conjugated antibody assay format 1.
The DXd conjugated Trastuzumab Sandwich ELISA assay was developed using DXd Antibody (39F4), mAb, Mouse (GenScript, A02217) and Mouse Anti-Human IgG Fc Antibody (50B4A9)[HRP], mAb (GenScript, A01854) as the capture and detection antibodies, respectively.
The samples tested in this assay were DXd conjugated Trastuzumab. The control samples were not serum spiked. The monkey serum samples were spiked with cynomolgus monkey serum matrix.
-
Total Antibody measurement by anti-idiotype antibody pairs
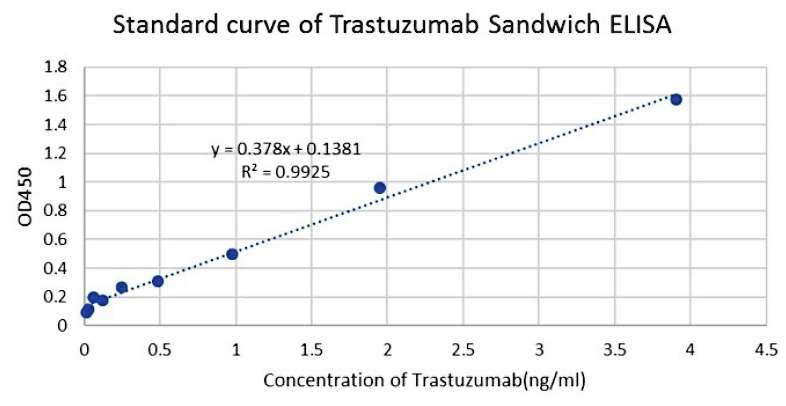
Figure 5. Standard curve of Trastuzumab Sandwich ELISA. The Trastuzumab Sandwich ELISA assay was developed using Anti-Trastuzumab Antibody (11C4), mAb, Mouse (GenScript, A02032-40) and Anti-Trastuzumab Antibody (15H2), mAb, Mouse (GenScript, A02033-40) as the capture and detection antibodies, respectively. Based on total antibody assay format 4.
In this ELISA assay, Anti-Trastuzumab Antibody (15H2), mAb, Mouse (GenScript, A02033-40) was labeled with Biotin.
GenScript can provide customized conjugation services for this product per the customer's request. The sensitivity of detecting Trastuzumab was observed to be up to 61 pg/ml.
Related Services
 Anti-idiotype Antibody Services
Anti-idiotype Antibody ServicesGuaranteed application in immunoassay development for PK and ADA analysis
Learn More Recombinant Protein Expression Service
Recombinant Protein Expression ServiceOffer high yield and cost-effective protein expression services in three main systems – Mammalian cells, Bacteria, and Baculovirus/insect
Learn More Hi!Ask me about GenScript services and products! I can answer questions or connect you to a live person.
Hi!Ask me about GenScript services and products! I can answer questions or connect you to a live person. -




































