-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- Pharmacokinetics and Immunogenecity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Impurity Test ELISA Kits
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitNew!
- -- Protein A ELISA KitNew!
- -- His tagged protein detection & purification
- -- dsRNA ELISA Kit
- -- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
Webinars » Bispecific Antibodies: The Next Generation of Antibody Therapeutics
Bispecific Antibodies: The Next Generation of Antibody Therapeutics
Within the past 3 years alone, the FDA has approved 20 novel antibody therapeutics for oncological treatment, with over 300 therapeutic antibodies currently in oncology based clinical trials . However, despite this exponential growth, most therapeutic antibodies rely on a monomeric immunotherapy based mechanism. Therefore, there is an urgent demand for new therapeutic strategies, such as combinatorial therapy, and novel modalities, such as bispecific antibodies.
In this webinar, we will cover
An overview of therapeutic antibodies, focusing on the opportunities and challenges of current monotherapy.The major benefits of bispecific antibodies and the current formats used in clinical trails.An in depth , integrated SMAB (single-domain antibody fused to monoclonal antibody) case study from rational design to preclinical development.
Webinar Details
Date: July 11, 2018 Time1: 8:00-9:00 A.M. GMT Time2: 1:00-2:00 A.M. GMT Speaker: Dr. Liusong Yin Speaker Bio
 Dr. Liusong Yin
Dr. Liusong Yin
Dr. Liusong Yin is the Director and Head of Antibody Department at GenScript USA Inc. Dr. Yin received his Ph.D. from the University of Massachusetts Medical School, specializing in antigen presentation, CD4+ T cell epitope selection, and antibody responses. Dr. Yin has extensive experience in Antibody drug discovery, he has published over 14 highly-cited papers in immunology, virology, and biochemistry Journals, and has received 5 patents in the antibody discovery field.
Related Services
Speaker-related Webinars

Identify the optimal protein purification strategy for your recombinant protein production
Are you challenged with obtaining sufficient amount of high purity proteins for your applications? Almost unavoidably, every protein purification step results in some degree of protein loss.

Recombinant protein expression & purification
Are you struggling with the amount and/or purity of your recombinant protein? Attend this online seminar for solutions to the challenges in recombinant protein expression & purification.

Accelerating Lead Identification Through Paramagnetic Bead Purification Technology
High throughput purification is a critical step in antibody engineering and lead identification. Paramagnetic bead technology allows a single step purification of targets by simply adding the beads directly
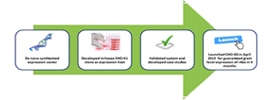
Efficient stable cell pool/cell line development for large-scale antibody and protein production
Interested in rapid and efficient stable cell pool/cell line development for large-scale production of your recombinant antibodies and proteins? If so, this webinar may be for you.

Chaperone co expression strategies for recombinant soluble protein production in E. Coli
Recombinant protein expression in E. coli often results in the formation of insoluble aggregates called inclusion bodies.

-







































