-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
Resources » Weekly Scientific Insight » Enhancing Cancer Immunotherapy: The Promising Role of circRNA in CAR T-Cell Therapy
scott.pritchett
Enhancing Cancer Immunotherapy: The Promising Role of circRNA in CAR T-Cell Therapy

Author: Dr. Tong Huang
June 13, 2024
What is CAR T-cell therapy?
CAR T-cell therapy is a personalized form of immunotherapy that uses altered T cells to selectively target and eliminate tumor cells. T cells from the patient are engineered to express chimeric antigen receptors (CAR) that recognize and bind to specific antigens on the surface of cancer cells. Since its first approval in the US in 2017, this form of immunotherapy has continued to gain attention and encourage researchers to apply the strategy throughout oncology and beyond.
Despite the recent successes of CAR T-cell therapies, researchers developing treatments within this category face a variety of challenges. While CAR T-cell therapies have proven to be effective in patients with certain blood cancers, efficacy against solid tumors has been limited. Also, because the manufacturing process requires T cell isolation, activation, engineering, expansion, and reinfusion for each patient, producing current CAR T-cell therapies is time-consuming and expensive. Because most CAR T-cell therapies currently on the market use viral vectors such as lentiviral/ retroviral vectors to insert CAR coding sequence into the genome of T cells, permanent genome integration and long-term gene expression raise safety concerns. Other non-viral platforms like the Sleeping Beauty transposon system and CRISPR gene editing technologies have also been used to introduce the CAR construct in the T cells, but the editing efficiency and integration stability can vary significantly.
Deployment of mRNA in CAR T-cell therapy
To mitigate these concerns, researchers are exploring the use of linear mRNA to create CAR T cells. This approach avoids permanent changes to the genome as mRNA degrades naturally over time, leading to only transient expression of the CAR protein. Delivery methods for mRNA include electroporation and nanoparticle systems. While electroporation is performed ex vivo, mRNA delivery by carriers such as lipid nanoparticles (LNPs), polymeric nanoparticles, and exosomes enables the generation of CAR T cells inside the patient. Generating CAR T cells in vivo using this delivery method avoids costly the manufacturing process required to produce CAR T cells ex vivo, improves safety, and enables rapid scalability.
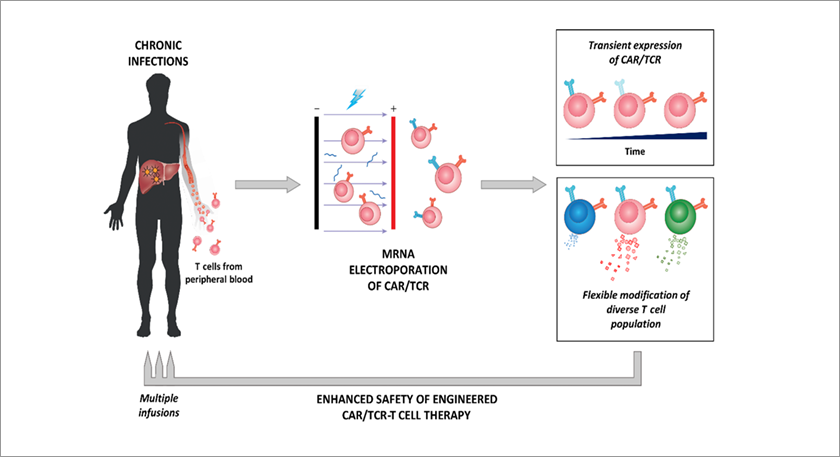
Transient expression of transgenic CAR/TCR through mRNA electroporation
(Image credit: Journal of Experimental Medicine)Nanoparticulate mRNA vaccines can also be designed to deliver CAR antigens to antigen presenting cells, stimulate adoptively transferred CAR T cells, and improve the therapeutic efficacy of CAR T cells. In the first-in-human phase 1/2 basket trial exploring CLDN6-specific CAR T-cell therapy combined with such a CAR T cell-amplifying mRNA vaccine, “encouraging signs of efficacy and disease control in a heterogenous cohort of hard-to-treat solid tumor patients” were observed.
Application of circRNA in CAR T-cell therapy
While the use of linear mRNA allows for transient expression of CAR, the expression is limited by mRNA’s susceptibility to exonuclease degradation. circular RNAs (circRNAs), covalently closed single-stranded RNA molecules that arise from back-splicing, are being considered to overcome this limitation. While most eukaryotic circRNAs are noncoding RNAs, some endogenous circRNAs and synthetic protein-coding circRNAs containing internal ribosome entry sites (IRESs) can be designed to express CAR and be used for CAR T-cell therapy. Due to the covalently closed continuous loop structure, circular RNAs are more stable than linear mRNA and enable consistent and prolonged expression of CARs.
Orna Therapeutics has reported promising preclinical results with their circRNA constructs, showing effective tumor control at low doses. In vivo tests showed that their optimized LNP-oCAR constructs had improved efficacy and tumor control at doses as low as 0.1 mg/kg. The lead ORN-101 effectively controls leukemia growth in mouse models at low doses when dosed every other week. These preclinical results show that circRNA-based approaches have promising potential to enable transient, redosable, and scalable CAR T-cell therapies.
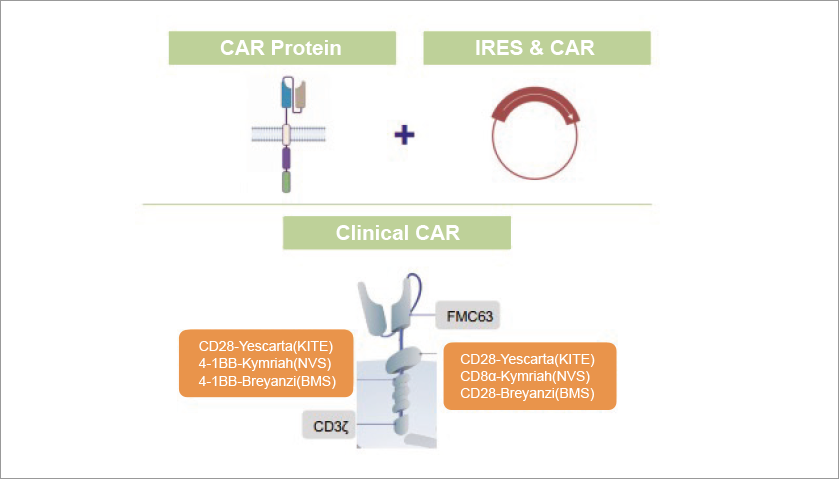
CAR oRNA Design
(Image Credit: Orna Therapeutics)Additionally, circRNAs also play important roles in physiological and pathological processes, including tumor immunity. Therefore, circRNAs have the potential to be promising targets or adjuvant for immunotherapy, and could improve the efficacy of CAR T cells.
Challenges of using CircRNA for CAR T-cell therapy
Despite the potential of circRNA-based approaches, several challenges need to be addressed to realize the full potential of circRNAs in CAR T-cell therapy. First, large-scale production of high-quality circRNAs with high purity is challenging. Circularization reactions are complex, and inefficiency in circularization results in side products, decreasing the purity of the final product. Multiple purification steps are required to remove impurities in the circularization crudes and therefore limit the overall yield of circRNA production. Targeted delivery systems to T cells must also continue to be optimized to enhance the efficacy and safety of circRNA-based therapies. While some preclinical data are available for CAR T-cell therapy using circRNA, more research, including preclinical and clinical trials, is needed to establish the safety, efficacy, and consistency of this approach.
Future prospects of circRNA application in CAR T-cell therapy
CAR T-cell therapy is a novel paradigm for cancer immunotherapy and RNA-based approaches may provide improve clinical outcomes through controlled and temporary expression and repeated dosing and fine-tuning of CAR expression levels. Due to their relatively high stability, circRNAs can offer more consistent and prolonged transient expression of CARs compared to linear mRNA. Combined with optimized delivery methods such as LNPs, CAR-encoding circRNAs can be delivered to T cells efficiently with enhanced stability and improved transfection efficiency.
Continued research in circRNA design, delivery methods, and clinical trials is essential to improve outcomes, reduce costs, and ensure safety. Despite the challenges, advances in circRNA applications hold the promise of transforming CAR T-cell therapy and expanding the range of cancers that can be treated.
References:
[1] Wu J, Wu W, Zhou B, et al. Chimeric antigen receptor therapy meets mRNA technology[J]. Trends in Biotechnology, 2023.
[2] Metanat Y, Viktor P, Amajd A, et al. The paths toward non-viral CAR-T cell manufacturing: A comprehensive review of state-of-the-art methods[J]. Life Sciences, 2024: 122683.
[3] Reinhard K, Rengstl B, Oehm P, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors[J]. Science, 2020, 367(6476): 446-453.
[4] Mackensen A, Haanen J B A G, Koenecke C, et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial[J]. Nature Medicine, 2023, 29(11): 2844-2853.
[5] Yu L L, Xiao Q, Yu B, et al. CircRNAs in tumor immunity and immunotherapy: Perspectives from innate and adaptive immunity[J]. Cancer Letters, 2023: 216219.
[6] Zhou Z, Sun B, Huang S, et al. Roles of circular RNAs in immune regulation and autoimmune diseases[J]. Cell death & disease, 2019, 10(7): 503.
[7] Emmanuel A, Becker A M, Wesselhoeft A, et al. in Situ CAR Therapy Using oRNA Lipid Nanoparticles Regresses Tumors In Vivo. ASGCT Annual Meeting, 2023.
[8] Anthony Tanoto Tan and Antonio Bertoletti (2020). Challenges of CAR- and TCR-T cell–based therapy for chronic infections. Journal of Experimental Medicine.
Subscribe to have the latest weekly scientific insights delivery to your inbox!
* We'll never share your email address with a third-party.

-








































