HOME
-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
![FLASH Gene]() FLASH Gene
FLASH Gene
Learn More
![Discover how TurboCHO™ delivers your antibody in just 5BDs!]() TurboCHO™ Antibody Expression
TurboCHO™ Antibody Expression
Learn More
-
![FLASH Gene]() FLASH Gene
FLASH Gene
Learn More
-
![Discover how TurboCHO™ delivers your antibody in just 5BDs!]() TurboCHO™ Antibody Expression
TurboCHO™ Antibody Expression
Learn More
-
![Custom
Monoclonal
Antibodies]() Custom Monoclonal Antibodies
Custom Monoclonal Antibodies
-
![Synthetic sgRNA Service]() Synthetic sgRNA Service
Synthetic sgRNA Service
-
![DNA Mutant Library]() DNA Mutant Library
DNA Mutant Library
-
 Catalog IVT mRNA and circRNA Products
Catalog IVT mRNA and circRNA Products
-
![siRNA Guarantee Package]() siRNA Guarantee Package KD%≥80%
siRNA Guarantee Package KD%≥80%
-
![Peptide
Synthesis Services]() TurboTide Service
TurboTide Service
Learn More
-
![Lentivirus Packaging Service]() Lentivirus Packaging Service
Lentivirus Packaging Service
-
-
PRODUCTS
-
Most Popular Products
-
CRISPR Gene Editing
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein Purification
-
Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Therapy
-
 Diagnostics
Diagnostics
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Cas
Nucleases]() Cas Nucleases
Cas Nucleases
-
![Anti-Camelid
VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![AAV2 and AAVX Titer Capsid ELISA Kits]() AAV2 and AAVX Titer Capsid ELISA Kits
AAV2 and AAVX Titer Capsid ELISA Kits
-
![Precast Gels]() Precast Gels
Precast Gels
-
![Protein
Isolation &
Purification]() Protein Isolation & Purification
Protein Isolation & Purification
-
![Recombinant Cytokines]() Recombinant Cytokines
Recombinant Cytokines
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro Customer Testimonial
AmMag™ Quatro Customer Testimonial
-
![Claudin 18.2]() Claudin 18.2
Claudin 18.2
-
![Cell Isolation]() Cell Therapy Comprehensive Product Solutions
Cell Therapy Comprehensive Product Solutions
-
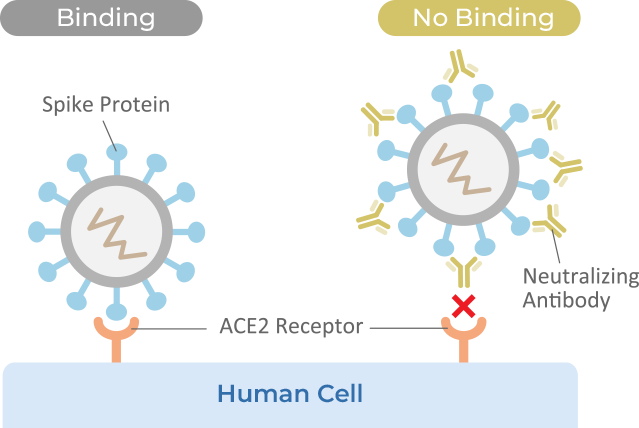
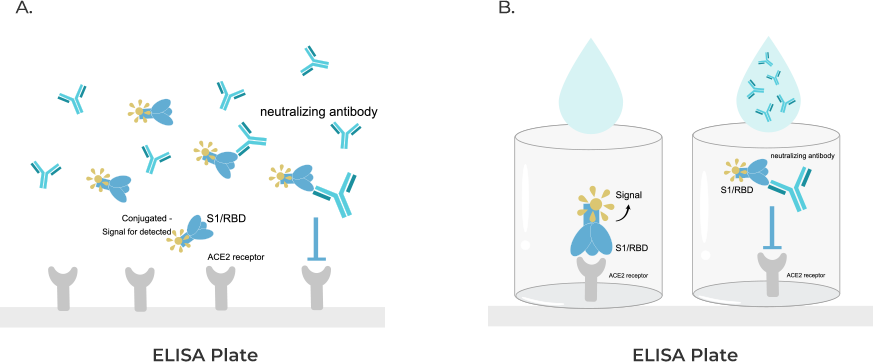
 cPass™ Technology RUO Kit
cPass™ Technology RUO Kit
-
![Quick
Order]() Quick Order
Quick Order
-
![Endotoxin
Detection &
Removal
System]() Endotoxin Detection & Removal System
Endotoxin Detection & Removal System
-
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER