-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
Resources » Learning Center » Research Digest » Engineering Extracellular Vesicles for Targeted Ovarian Cancer Therapy | GenScript
Engineering EVs for Ovarian Cancer Targeting
Ovarian cancer therapeutic challenges
Ovarian cancer remains among the ten most diagnosed malignancies globally, with a high mortality rate. In 2020, over 200,000 deaths were attributed to this malignancy worldwide. Because ovarian cancer frequently progresses to late stages before diagnosis, it has a low survival rate, below 50%.1
Conventionally, ovarian cancer treatment involves surgery followed by chemotherapy with taxanes and platinum-based drugs.2 Most patients, ~80%, show complete responses to this first treatment course and remain disease-free for over one year. However, disease recurrence is frequent, especially for those with advanced disease, necessitating a second course of chemotherapy. At this stage, drug resistance and toxicities are major challenges for re-implementing chemotherapy.1,2
Paclitaxel is a plant-derived taxane frequently used as a chemotherapeutic agent in ovarian cancer.3 Despite being an effective therapy for various cancer indications, its generalized mechanism of action (i.e., microtubule stabilization, mitotic arrest, and cell proliferation inhibition) results in off-target toxicities following systemic administration. Several strategies have been implemented to enable a more tumor-targeted effect, including its conjugation to small molecules, antibodies, or aptamers and the encapsulation of Paclitaxel in nanoparticles.4
Advantages of nanoparticles in chemotherapy delivery
The use of nanoparticles for the delivery of Paclitaxel and other chemotherapy drugs provides several benefits, including the potential for specific tumor targeting, controlled release, and improved stability. Stable protein-based synthetic nanoparticles encapsulating Paclitaxel proved effective in a glioma-bearing mouse model, extending survival and reducing tumor size while preventing liver toxicity.5
Naturally derived vesicles or extracellular vesicles, such as exosomes, can also be leveraged in chemotherapy drug delivery. Extracellular vesicle nanoparticles offer various benefits as delivery vehicles, including low toxicity and immunogenicity and the capacity to traverse physiological barriers of interest, such as the blood-brain barrier. Previously, exosomes derived from brain endothelial cells were shown to serve as an efficient vehicle to deliver chemotherapy drugs in a zebrafish brain cancer model, leading to tumor cytotoxicity.6
Engineered extracellular vesicles for ovarian cancer
Beyond their favorable physiological properties, extracellular vesicles can be modified further to make them even more useful for cancer drug delivery. In new work led by Professor Carlos Salomon Gallo at the Centre for Clinical Research, University of Queensland, extracellular vesicles have been engineered to achieve improved cargo delivery to ovarian cancer cells.2
In their new publication, Alharbi and colleagues have engineered extracellular vesicles to present the ligand for the ephrin-B4 receptor, ephrin-B2, on the surface. Briefly, extracellular vesicles were prepared from HEK293T cells transfected with a plasmid encoding LAMP2b fused to GFP and ephrin-B2. Once expressed, the chimeric membrane-bound LAMP2b protein supported intra- and extra-vesicular GFP and Ephrin-B2 expression, respectively.
Extracellular vesicles were isolated from the cell culture supernatant through differential centrifugation and size exclusion chromatography, followed by various analyses to validate their expected properties, such as their size and expression of various markers (e.g., CD63, CD81, and CD9). Additionally, the expression of ephrin-B2 was confirmed via MS/MS analysis.
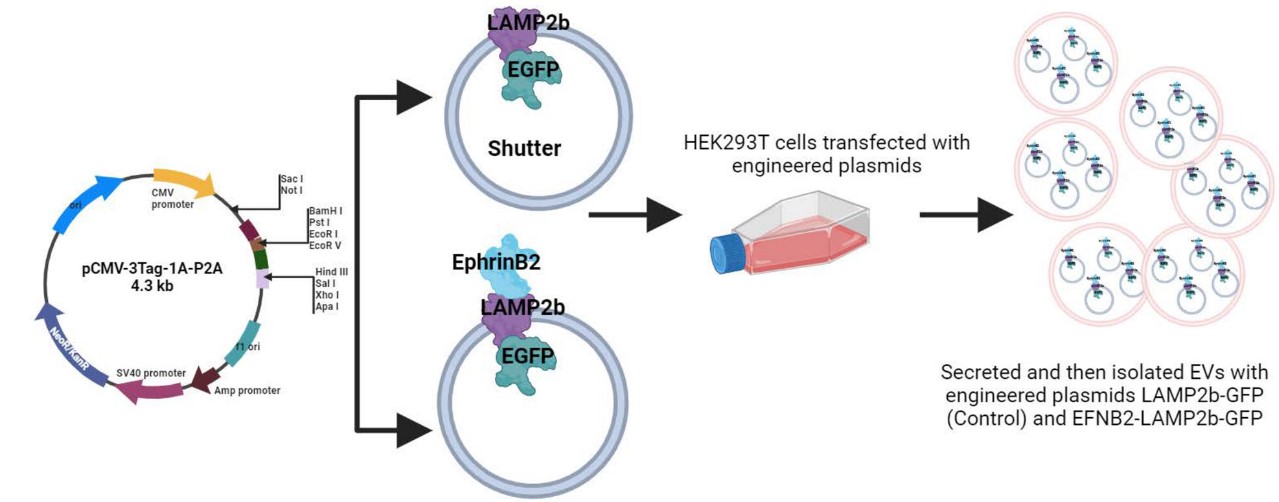
“Two expression vectors were constructed: a control vector that expresses LAMP2b with a C-terminally fused enhanced green fluorescent protein (EGFP; hereafter, simply referred to as GFP), and one that expresses LAMP2b with a C-terminally fused GFP and an N-terminally fused ephrin-B2 (EFNB2) peptide for targeting the ephrin-B4 receptor. Each construct (LAMP2b-GFP and EFNB2-LAMP2b-GFP) was cloned into a pCMV-3 Tag-1A-P2A expression vector backbone, downstream of the CMV promoter between NotI and ApaI restriction sites (Vectors were designed in house and generated by Genscript).”2Retrieved from Alharbi et al. 2024, only a portion of the abstract figure is shown. Deed - Attribution 4.0 International - Creative Commons
in vitro and in vivo uptake of extracellular vesicles
By decorating the surface of extracellular vesicles with ephrin-B2, the team hoped to improve their uptake via receptor-mediated endocytosis by ovarian cancer cells, which overexpress the ephrin-B4 receptor. Internalization of extracellular vesicles expressing ephrin-B2-LAMP2b-GFP and those only expressing the control LAMP2b-GFP was evaluated first in vitro, using cultured ID8 mouse ovarian cancer cells. The team found that both vesicles were similarly internalized by ID8 cells in culture. In contrast, following intravenous injection of extracellular vesicles in an ID8 ovarian tumor-bearing mouse model, the internalization of ephrin-B2-LAMP2b-GFP extracellular vesicles was significantly higher than the control within the tumor.
Not surprisingly both types of extracellular vesicles were also internalized to a similar extent in various organs (e.g., spleen, brain, and lung). Additionally, as is the case with many different delivery vehicles, including lipid nanoparticles (LNPs), the highest internalization of extracellular vesicles occurred in the liver.
Conclusions
In their study, the University of Queensland team investigated the potential of extracellular vesicles with surface expression of ephrin-B2 for targeting ovarian cancer cells. Their in vivo findings suggest that these engineered extracellular vesicles could offer a targeting advantage. However, the authors note that using extracellular vesicles for delivering therapies is a very complex process. One significant challenge is the absence of standardized workflows based on clinical regulatory guidance for large-scale production of optimized extracellular vesicles.
Reference
1. Tavares, V. et al.,(2024) A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. https://doi.org/10.3390/ijms25031845
2. Alharbi, M. et al., (2024) Enhancing precision targeting of ovarian cancer tumor cells in vivo through extracellular vesicle engineering. International Journal of Cancer. https://doi.org/10.1002/ijc.35055
3. Ying, N. et al., (2023). Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids and Surfaces B: Biointerfaces, https://doi.org/10.1016/j.colsurfb.2023.113419
4. Ma, Y. et al., (2021) Targeting Strategies for Enhancing Paclitaxel Specificity in Chemotherapy. Front Cell Dev Biol. https://doi.org/10.3389%2Ffcell.2021.626910
5. Mauser, A. et al., (2024) Controlled Delivery of Paclitaxel via Stable Synthetic Protein Nanoparticles. Advanced Therapeutics, 2400208. https://doi.org/10.1002/adtp.202400208
6. Yang, T. et al., (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. https://doi.org/10.1007%2Fs11095-014-1593-y

-