-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
News & Blogs » Peptide News » Neoantigen peptides and T-scan: high output screening platform with GenScript peptide service
Neoantigen Peptides and T-scan: High output Screening Platform with GenScript Peptide Service
Neoantigen and T Cell Epitope Mapping
Neoantigens, also known as tumor-specific antigens, is a class of HLA-bound peptides generated by tumor-specific mutations. They are not present in normal tissues, thus can be used as biomarkers differentiating cancer cells from normal cells. Compared to those non-mutated self-antigens, neoantigens could be recognized as non-self by the host immune system and are thus attractive targets for immunotherapies with potentially increased efficacy, specificity, and safety. It has been shown that recognition of these individual-specific neoantigens opens a new door for cancer immunotherapy.
The immunogenicity of neoantigens leading to T cell response has long been demonstrated in human. Recently, independent clinical studies provided solid evidence that neoantigen-based cancer vaccines could be developed to elicit potent neoantigen-specific T cell responses against late stage melanoma with remarkable safety and efficacy. These and other recent advances (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6036114/table/T1/?report=objectonly) have triggered the enthusiasm in pursuing cancer vaccines against neoantigens.
However, one of the key challenges in the development of neoantigen-based therapies is that immunogenic neoantigens are rare and difficult to predict. And the TCR signaling is complex as antigen binding does not uniformly lead to functional TCR signaling. Thus, accurate predictive algorithm and validation tools are need to be optimized for accurate prediction of binding peptides and reliable selection of highly immunogenic neoepitopes. Classic approaches for understanding T cell specificity and possible neoantigen rely on readouts of T cell function, which include assays for cytotoxicity, cytokine release, and proliferation in the presence of candidate's antigens, augmented by peptide-MHC tetramers for antigen-specific populations and others. However, these are primarily useful for predetermined sets of 10-100 s of antigens but are unsuitable at genome scale.
Several other approaches have been taken to map unknown T cell specificities and neoantigens. A recent approach uses display of peptides as single-chain fusions to MHC on the surface of target cells. T cell binding to cognate antigen results in trogoctyosis or activation of a synthetic signaling molecule, enabling the isolation of recognized target cells. Another method uses display of genetically encoded random peptides covalently attached to MHC molecules on the surface of yeast and use soluble TCRs to pan the yeast library for peptide-MHC binding. Notably, neither yeast display nor single-chain approaches require endogenous processing of antigens nor functional activation of T cells, which leaves uncertainty as to whether the identified antigens are physiologically meaningful in vivo.
T-Scan
To overcome such obstacle, a new platform named T-Scan was developed. It is a cell-based pooled screen, high-throughput, genome-wide platform for the systematic identification of antigens by T cells. This platform harness lentiviral delivery of antigen libraries into cells for endogenous processing and presentation on major histocompatibility complex (MHC) molecules, correctly identifies cognate antigens of T cell receptors (TCRs) from viral and human genome-wide libraries. The antigen recognition by TCRs is monitored through a fluorescent reporter of activity in the target cells, hereby characterize the reactivity of a tumor-derived TCR.
(image resource from https://www.sciencedirect.com/science/article/pii/S0092867419307743#undfig1)
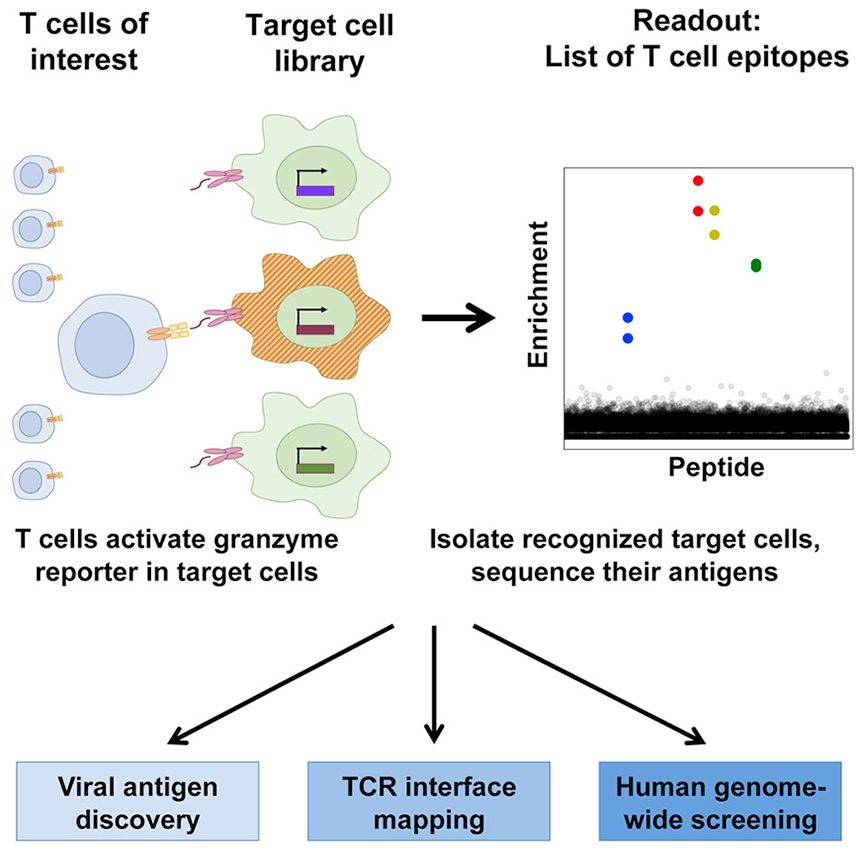
Genscript's Peptide for T-scan and Neoantigen Service
Peptides can be used as single stimulants, pools or libraries, or as part of peptide/major histocompatibility complexes (MHC) for direct T-cell receptor staining and related therapeutic application. For stimulating T cells, peptides must be bound to MHC molecules. Thus the quality of synthetic peptide is crucial on this contribution. Based on theoretic considerations, published data, feedback from customers and our over 10-years experience, we have set a strict quality standards for neoantigen peptides, including peptide purity, TFA control, TFA removal, endotoxin control, endotoxin removal and so on. These parameters contribute to effective stimulating while reduce interference factors. Besides, considering the neoantigen peptide hydrophobicity, our solubility test facilitate customer to choose proper solvent hereby ensuring the usability. These considering service attracts more clients to cooperate with Genscript, covering from fundamental researches to preclinical trials.
-
T-Scan: A Genome-wide Method for the Systematic Discovery of
T Cell Epitopes. Cell. (2019).
DOI: https://www.sciencedirect.com/science/article/pii/S0092867419307743
First Author Conversations Podcast
Anna Pasetto, Ph.D., Karolinska Institutet
Learn MoreSubscribe to Receive Updates
& Promotions From GenScript* We'll never share your email address with a third-party.
Latest News & Blogs
Find More Peptide News
The Future of Neoantigen Vaccines: How Nanoparticles, Liposomes, and Viral Vectors Are Revolutionizing Treatment as Novel delivery System
Sep 22, 2025

Unveiling the Potential of GLP-1 Receptor Agonists and Multi-Target Therapies
Jul 07, 2025

Challenges in Neoantigen Peptide Manufacturing | GenScript
Apr 28, 2025

Challenges & Innovations in Neoantigen Peptide Vaccine Production
Apr 02, 2025

The Clinical Application of Neoantigens: A Promising Frontier in Cancer Immunotherapy
Feb 25, 2025

Delving into the World of Cyclic Peptides: Harnessing the Power of Circular Molecules for Innovative Therapeutic Solutions
May 14, 2024

-







































