-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![Neoantigen Peptide Synthesis Service Neoantigen Peptide Synthesis Service]()
Neoantigen Peptide Synthesis Service
Reliable Neoantigen Peptides for Precision Therapeutic Discovery
News & Blogs » Peptide News » How the Proteasome Modulates the Immunopeptidome
How the Proteasome Modulates the Immunopeptidome
The immunopeptidome is the repertoire of short peptides presented by the Major Histocompatibility Complex class I and class II (MHC I and MHC II) transmembrane proteins. Several key features distinguish these two antigen presentation pathways. For example, MHC class I proteins are ubiquitously expressed by all nucleated cell types, while MHC class II proteins are only expressed by immune antigen-presenting cells (APCs). Peptides loaded onto MHC I proteins (8-10 amino acids) are processed by the proteasome; in contrast, peptides loaded onto MHC II proteins (10-25 amino acids) undergo proteolysis within lysosomes or phagosomes. Finally, MHC I and MHC II bound peptides are specifically recognized by CD8 and CD4 T cells, respectively.
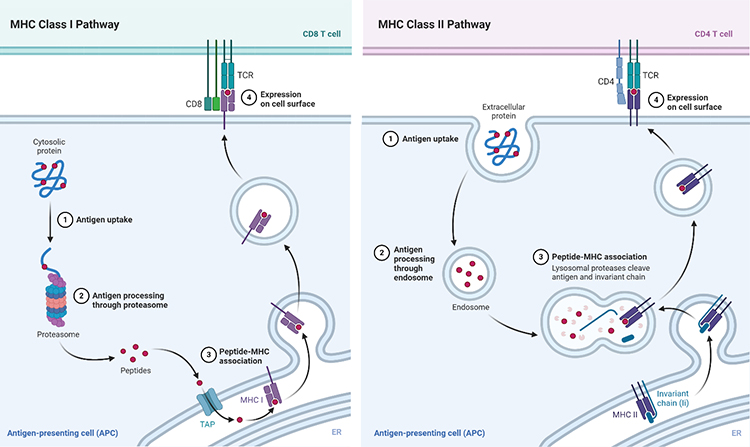
Reprinted from “MHC Class I and II Pathways”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates
Both MHC I and MHC II class peptides may be leveraged for cancer immunotherapy strategies (Janeway et al. 2001). These strategies are based on identifying novel antigens or “neoantigens,” which arise in cancer cells in association with somatic mutations. For example, identified MHC I neoantigen peptides may support the development of personalized vaccines to promote CD8 T cell-mediated tumor cell killing (Bianchi et al. 2020).
Multiple Proteasomes Shape the MHC I Immunopeptidome
The proteasome is a multi-protein complex, consisting of a 20S core catalytic unit and associated regulatory subunits, such as 19S, PA28, and PA200. Together, this complex mediates ubiquitinated-protein degradation. The proteasome’s activity is not only determined by associated regulatory subunits but is also influenced by its 20S core components. Several 20S core subsets, which differ in β subunit composition, give rise to constitutive, immune, and intermediate proteasomes. Among these, the immunoproteasome predominates in lymphoid tissues. However, in non-immune cells, proinflammatory cytokines such as IFN (α, β and γ) and TNF induce the immunoproteasome (Murata et al. 2018, Kors et al. 2020). Because tumors possess an inflammatory microenvironment, a standing question in the field pertains to the role of inflammation in modulating the proteasome’s composition and activity, and consequently, how it shapes peptide presentation and the immunopeptidome.
Lessons from the Melanoma Immunopeptidome
The influence of proinflammatory cytokines in modulating the melanoma immunopeptidome has been more recently highlighted by Faridi et al. 2020. In this study, investigators isolated HLA class I bound peptides from a melanoma cell line untreated or treated with IFNγ. Investigators identified two main groups of HLA class I bound peptides, linear (>90%) and cis-spliced peptides (~7%), irrespective of INFγ treatment conditions. Consistent with previous work, treatment with INFγ induced the immunoproteasome and led to an expanded immunopeptidome, providing the opportunity to identify new epitopes with therapeutic potential.
Significantly, investigators identified changes in the composition of the immunopeptidome, indicated by changes in the proportion of specific melanoma-associated peptides in the presence of INFγ. Additionally, the study was successful in identifying new cis-spliced immunogenic melanoma epitopes. Because some of these newly identified cis-spliced peptides were recognized in vitro by T cells derived from different melanoma patients, investigators are hopeful for their potential as targets for immunotherapy.
Reference
- Bianchi, V., Harari, A. & Coukos, G. Neoantigen-Specific Adoptive Cell Therapies for Cancer: Making T-Cell Products More Personal. Frontiers in Immunology (2020) doi:10.3389/fimmu.2020.01215.
- Faridi, P. et al. Spliced Peptides and Cytokine-Driven Changes in the Immunopeptidome of Melanoma. Cancer Immunol. Res. (2020) doi:10.1158/2326-6066.cir-19-0894.
- Kors, S., Geijtenbeek, K., Reits, E. & Schipper-Krom, S. Regulation of Proteasome Activity by (Post-)transcriptional Mechanisms. Front. Mol. Biosci. (2019) doi:10.3389/fmolb.2019.00048.
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Antigen recognition by T cells. Available from:https://www.ncbi.nlm.nih.gov/books/NBK27098/
- Murata, S., Takahama, Y., Kasahara, M. & Tanaka, K. The immunoproteasome and thymoproteasome: functions, evolution and human disease. Nature Immunology (2018) doi:10.1038/s41590-018-0186-z.
- Raghavan, M., Zaitoua, A. J. & Kaur, A. Variations in MHC class I antigen presentation and immunopeptidome selection pathways. F1000Research (2020) doi:10.12688/f1000research.26935.1.
Subscribe to Receive Updates
& Promotions From GenScript* We'll never share your email address with a third-party.
Latest News & Blogs
Find More Peptide NewsThe Future of Neoantigen Vaccines: How Nanoparticles, Liposomes, and Viral Vectors Are Revolutionizing Treatment as Novel delivery System

Unveiling the Potential of GLP-1 Receptor Agonists and Multi-Target Therapies

Challenges in Neoantigen Peptide Manufacturing | GenScript

Challenges & Innovations in Neoantigen Peptide Vaccine Production

The Clinical Application of Neoantigens: A Promising Frontier in Cancer Immunotherapy

Delving into the World of Cyclic Peptides: Harnessing the Power of Circular Molecules for Innovative Therapeutic Solutions


-






































