-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
Resources » Learning Center » Research Digest » Maximizing Gene Editing Efficiency with Optimized LNP-Based Delivery | GenScript
Maximizing Base and Prime Editing Efficiency with Optimized LNP-Based Delivery
Gene editing continues to revolutionize medicine, offering potential cures for genetic disorders and possibly chronic diseases by precisely modifying genome sequences. However, for this technology to realize its full potential, efficient delivery of gene-editing tools—such as CRISPR/Cas9 proteins and guide RNAs—is essential. A groundbreaking study published in Nature Biomedical Engineering introduces an innovative approach to enhancing the safety and efficiency of these tools by optimizing the delivery of ribonucleoproteins (RNPs) through lipid nanoparticles (LNPs).1
Evolving gene editing tool delivery approaches
Traditionally, viral vectors like adenovirus, adeno-associated virus (AAV), and lentivirus have been the go-to options for gene-editing delivery due to their high transduction efficiency. However, these vectors come with limitations:1-3
- Restricted Packaging Capacity: Large gene-editing tools, such as Streptococcus pyogenes Cas9 (SpCas9), can exceed the size limits of some viral vectors.
- Safety Concerns: The risk of genomic integration and insertional mutagenesis poses significant therapeutic challenges.
- Prolonged Expression Risks: High and sustained expression of editors increases the likelihood of immunogenic reactions and off-target effects.
Key Properties of Various Viral and Non-Viral Gene Editing Delivery Vehicles
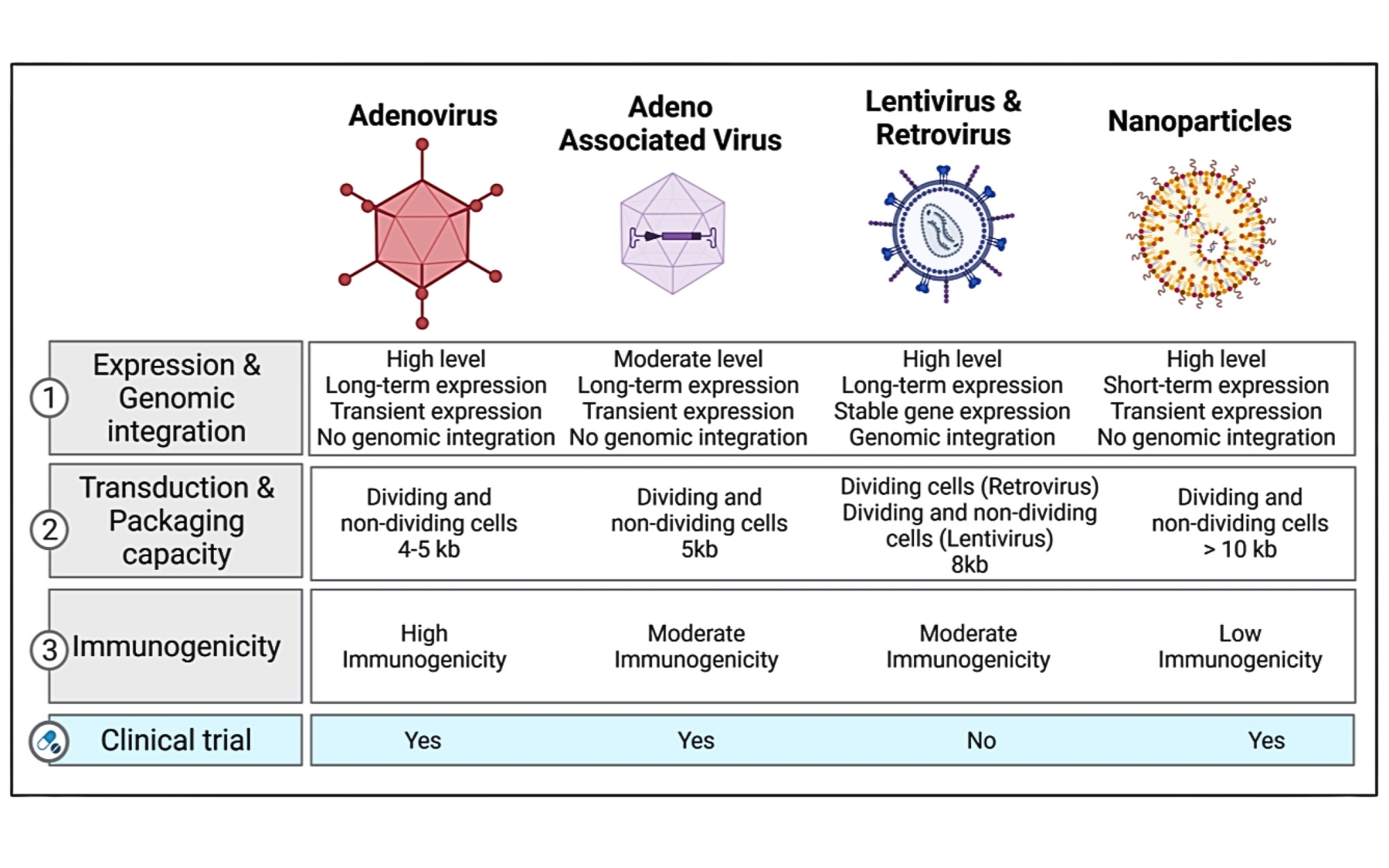
Retrieved with modifications from Bisserier et al. 2022, Figure 2.2
To address these issues, researchers are turning to non-viral delivery methods, including lipid and virus-like nanoparticles.1,4 These methods allow for greater packaging capacity and the delivery of fully functional CRISPR/Cas9 RNPs, minimizing risks like insertional mutagenesis and prolonged expression of editors. However, achieving high efficiency and precision, particularly for in vivo applications, remains a significant hurdle.
Improving editing precision with new Cas9 proteins
New strategies using advanced Cas9 proteins, such as base editors (e.g., adenine base editors or ABEs) and prime editors (PEs), are redefining the landscape of gene editing. Unlike conventional Cas9-based editing, which relies on double-strand breaks (DSBs) and repair pathways, base and prime editors enable:1
- Precise Modifications: Base editors efficiently convert specific base pairs (e.g., C→T or A→G) without introducing DSBs.
- Versatility: Prime editors can perform base substitutions, insertions, and deletions with minimal off-target effects.
- Improved Safety: These approaches reduce unintended consequences like large insertions, deletions, or chromosomal rearrangements.
These innovations are particularly promising for developing gene-correcting strategies for monogenic diseases.
Leveraging cell penetrating peptides to improve RNP delivery efficiency
In the recent Nature Biomedical Engineering publication, Hołubowicz and colleagues initially explored cell-penetrating peptides (CPPs) to enhance the delivery of gene-editing tools.1 By testing CPPs such as TAT, CPP5, and ANTP, the researchers aimed to improve RNP delivery to retinal pigment epithelium (RPE) and photoreceptors. While CPPs showed promise for certain proteins like Cre recombinase, they fell short for ABE and PE RNPs.
However, a non-covalent CPP, 6×His-CM18-PTD4, combined with 10% sucrose to stabilize proteins, improved delivery efficiency modestly. This approach demonstrated some restoration of retinal function in a Leber congenital amaurosis (LCA) mouse model but achieved limited editing efficiency (~2% correction in RPE).

Monitoring the effects of CPPs on ABE8e editing efficiency. (a) The fluorescent rd12 reporter cell line was developed based on the rd12 reporter construct synthesized by GenScript (mCherry-rd12-eGFP). In this cell line, correction of the rd12 mutation by ABE results in dual expression of mCherry and eGFP, facilitating the monitoring of ABE RNP delivery efficiency. (d) Effects of sucrose concentration and the non-covalent CPP 6×His-CM18-PTD4 (GenScript) on RNP delivery and editing efficiency. Fusion of CPPs to ABE8e failed at improving RNP delivery efficiency. Retrieved from Holubowicz et al. 2024 Figure 3, with modifications- only panels a and d are shown.1
Optimizing LNPs for ABE and PE delivery
Recognizing the limitations of CPPs, Hołubowicz and colleagues determined that delivery of ABE RNPs in vitro and even in vivo could be improved by simply using Lipofectamine 3000, but despite being an effective approach, in vivo, the reagent was toxic at some concentrations. Therefore, the team shifted focus to lipid nanoparticles, which could be fine-tuned to improve CRISPR RNP encapsulation, and provided a tried, tested and safe delivery vehicle.
Having optimized LNP lipid composition to safeguard ABE’s activity and eliminate any potential toxicity the team found that in vitro this new approach drove efficient delivery, resulting in dual expression of mCherry and eGFP in mostly all rd12 cells. More important to their ultimate goal, the ABE LNPs were safe and an efficient delivery vehicle in vivo, rescuing retinal responses significantly above of what they could achieve with Lipofectamine and approaching normal responses.
Fine tuning the lipid composition and specifically the concentration of DMG-PEG 2000 was critical to maximize RNP encapsulation, delivery and reassuringly to improve editing precision. Interestingly, the team found that LNP optimization is not a fit-for-all editing tools, as the optimized formulation for ABE had to be further fined tunned to achieve maximal encapsulation. Nevertheless, once optimized the LNP formulation enabled efficient delivery of PE RNPs, supporting high precision of editing in vitro and restoring in vivo retinal functionality on par with the rescue achieved with ABE RNP LNPs.
A leap forward in therapeutic applications
Hołubowicz and colleagues showcased a groundbreaking achievement: delivering a single dose of ABE or PE RNPs via their optimized LNP formulations led to remarkable genetic correction, driving measurable physiological and biochemical improvements. This innovation not only highlights the power of precise gene editing but also signals a leap forward in therapeutic applications.
Looking ahead, integrating cutting-edge strategies, like tissue-targeting antibodies with optimized LNPs can help redefining the limits of what’s possible in gene editing. These advancements open new frontiers for realizing the full therapeutic potential of gene editing, bringing us closer to transforming lives with precision medicine.
Reference
1. Hołubowicz, R., et al. (2024). Safer and efficient base editing and prime editing via ribonucleoproteins delivered through optimized lipid-nanoparticle formulations. Nature Biomedical Engineering. https://www.nature.com/articles/s41551-024-01296-2
2. Bisserier, M., et al., (2022). Novel insights into the therapeutic potential of lung-targeted gene transfer in the most common respiratory diseases. Frontiers in Pharmacology, 13, Article 956423. https://www.mdpi.com/2073-4409/11/6/984
3. Mengstie, M. A., (2022). Viral vectors for the in vivo delivery of CRISPR components: Advances and challenges. https://doi.org/10.3389/fbioe.2022.895713
4. Baskota, S., et al. (2023). Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. https://doi.org/10.1016/j.cell.2021.12.021

-





































