HOME
-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
CRISPR Gene Editing
-
Cell Engineering
-
- Custom Peptide Synthesis
- Catalog PeptidesHot!
- Neoantigen PeptideHot!
- Cosmetic Peptide Synthesis
- Peptide Modification
- Cyclic Peptide New!
- Peptide Applications
- Peptide Library
- Peptide Drug Conjugates Service New!
- cGMP Peptide Synthesis New!
- AccuPep+ QC
- Peptide Technology & Resource
- GLP-1 Receptor Agonist ServicesNew!
-
- Cas9 sgRNA Synthesis Service
- Cas12a (cpf1) crRNA Synthesis Service
- pegRNA Synthesis Service
- HDR Templates 30% off ssDNA!
- CAR-T Knock-in Optimization Kit
- cGMP sgRNA
- cGMP HDR Templates
- CRISPR Plasmids
- CRISPR gRNA Plasmid Library
- CRISPR Cell Engineering
- Microbial Genome Editing
- sgRNA + HDR Design Tool New!
- CRISPR/Cas Protein Production New!
-
-
PRODUCTS
-
Most Popular Products
-
CRISPR Gene Editing
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein Purification
-
Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Therapy
-
 Diagnostics
Diagnostics
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Cas
Nucleases]() Cas Nucleases
Cas Nucleases
-
![Anti-Camelid
VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![AAV2 and AAVX Titer Capsid ELISA Kits]() AAV2 and AAVX Titer Capsid ELISA Kits
AAV2 and AAVX Titer Capsid ELISA Kits
-
![Precast Gels]() Precast Gels
Precast Gels
-
![Protein
Isolation &
Purification]() Protein Isolation & Purification
Protein Isolation & Purification
-
![Recombinant Cytokines]() Recombinant Cytokines
Recombinant Cytokines
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro Customer Testimonial
AmMag™ Quatro Customer Testimonial
-
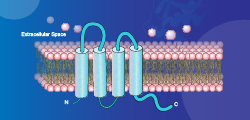
![Claudin 18.2]() Claudin 18.2
Claudin 18.2
-
![Cell Isolation]() Cell Therapy Comprehensive Product Solutions
Cell Therapy Comprehensive Product Solutions
-
 cPass™ Technology RUO Kit
cPass™ Technology RUO Kit
-
![Quick
Order]() Quick Order
Quick Order
-
![Endotoxin
Detection &
Removal
System]() Endotoxin Detection & Removal System
Endotoxin Detection & Removal System
-
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER