-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- Pharmacokinetics and Immunogenecity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Impurity Test ELISA Kits
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitNew!
- -- Protein A ELISA KitNew!
- -- His tagged protein detection & purification
- -- dsRNA ELISA Kit
- -- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![Lead Selection, Optimization & Characterization Lead Selection, Optimization & Characterization]()
Lead Selection, Optimization & Characterization
Resources » Technical Resource Centers » Antibody Drug Discovery Technical Resource » Lead Selection and Optimization
- Antibody Formats
- Antibody Drug Discovery Overview
- Target Identification and Validation
- Antibody Drug Discovery Webinars
- Protein Webinars
- Hit Generation and Screening
- Lead Selection and Optimization
- Candidate Selection
- Antibody Expression in E.coli
- Transient vs. Stable Expression
- Antibody Expression in Eukaryotic Hosts
Overview
What is Lead Selection in Antibody Drug Discovery?
Lead selection refers to the process by which the early hits are interrogated in a vigorous, multi-step screening process to select lead molecules that meet pre-established criteria for progressing to the next stage. Screening via secondary and tertiary functional assays filters from hundreds of hits down to a few Ab molecules. These are then purified in milligram quantities for more detailed characterization1.
Lead characterization typically includes in vitro efficacy studies for confirmation of binding and functional activities as well as biochemical and biophysical analyses. Common molecular analysis includes determination of expression levels from mammalian expression systems, aggregation analysis by SEC, SDS-PAGE, Western blot analysis, determination of target protein binding affinity by Biacore and KinExA analysis, and crude epitope mapping1. Examples of in vitro studies include Fcγ, FcRn, C1q assays. Upon completion of in vitro characterization, selected hits are ready for expression and purification in sufficient quantity (typically few hundred milligrams to grams) for in vivo efficacy testing. Occasionally, a crude PK study is also conducted prior to in vivo efficacy study to help establish the dosing regimen. Typically the lead molecule is selected based on demonstrated in vivo efficacy, which is often a go/ no-go decision point for the program1.
Lead Optimization & Characterization
Steps include Ab production, humanization (in case of rodent or other non-human antibody), affinity maturation, Fc engineering, characterization of biochemical properties, in vitro and in vivo pharmacology. Ab production is via transient expression or stable cell lines. in vivo pharmacology includes PoC efficacy, MOA, PK/PD and preliminary toxicological studies.
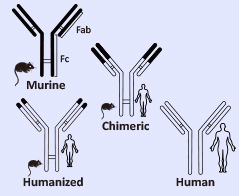
What is humanization and why is it performed?
Humanization is a technique to reduce the immunogenicity of a therapeutic antibody initially derived from rodents [non-humans]. The process refers to the replacement of more than 90% of rodent IgG sequence in the parental antibody molecule with human IgG sequence.
Figure 1: Schematic on right illustrates principle.What is affinity maturation and why is it performed?
Affinity maturation is usually applied to antibody leads selected from a naïve human library using a display technology. These leads may have relatively low (10–100 nM) target binding affinities but can be enhanced to reach a desired affinity range (normally 0.1–10 nM). Note that high affinity does not always correlate with improved Ab efficacy.
What is Fc engineering and why is it performed?
The Fc region of an Ab is a functional molecular entity mediating
- ADCC via binding to Fcc receptors (FccR) on natural killer (NK) cells
- CDC via C1q binding
- Increase in the in vivo half-life via binding to the neonatal Fc receptor (FcRn)
Alteration of each of these activities has been explored to modulate the function of Ab in specific applications. For example, ADCC enhancement improves tumor cell killing, achieved by engineering site-directed mutations in the contact residues. You could also increase Ab half-life by Fc engineering. After the generation of an optimized lead, further functional and molecular characterization is carried out to confirm its in vitro and in vivo activity and favorable molecular attributes as a therapeutic candidate1.
The previous section in this series is "Hit Generation and Screening". To review, click here
GenScript Antibody Drug Discovery Services
Antibody Discovery
GenScript’s Antibody Engineering group can build antibody library with up to 1010 individual clones, to speed up your antibody discovery efforts.
Antibody Sequencing
GenScript’s advanced Antibody Sequencing technology offers fast and professional sequencing services for your monoclonal antibodies.
Assays
GenScript has developed several cell-based ADCC/CDC functional assays to profile the efficacy and potency of your therapeutic antibodies using proprietary recombinant effector cells.
Antibody Engineering
GenScript scientists’ extensive experience in antibody engineering can provide superior services such as antibody humanization, affinity maturation and more.
Antibody Production
With solid expertise in recombinant antibody (rAb) production techniques, GenScript provides a comprehensive rAb service portfolio that deliver microgram to gram quantities of pure rAb for each stage of your Ab drug discovery program.
PK/PD Study
GenScript offers over 120 tumor and inflammation models for evaluation of in vivo efficacy, PK/PD, biomarker and bioanalysis studies. GenScript Anti-idiotype Antibody services are also a powerful tool for antibody drug PK/PD and immunogenicity studies.
You can also view our Recombinant Antibody Service Selection Guide to identify services that are the best match for your application.
References
- Shih, H. H. in Development of Antibody-Based Therapeutics (ed M.A; Bornstein Tabrizi, G.G.; Klakamp, S.L.) Ch. 2, 9-32 (Springer, 2012).
Quotations and Ordering
Click the quote button,provide service information and submitOur Technical Account Manager will contact you within 24 hours to finalize quoteYou review and approve final order details and priceYou provide credit card/PO information and order is plcaedProject is initiated immediatelyOur Project Manager will be in contact with you to provide updatesOur customer service repsentatives are available 24 hours a day, Monday through Friday, to assist you.

-





































