Glutamine Synthetase Expression Technology Principle

The Glutamine Synthetase Expression Technology is widely used for recombinant antibody and protein production in mammalian cell culture. Glutamine Synthetase Expression Technology relies on glutamine synthetase enzyme which synthesizes glutamine from glutamate and ammonium ions. Since many mammalian cells cannot make endogenous glutamine, transfection of such cells with a vector encoding Glutamine Synthetase enzyme ensures cell survival in culture. And for those cells that make endogenous glutamine, using the Glutamine Synthetase enzyme inhibitor, methionine sulfoximine (MSX), enables suppression of the endogenous glutamine. In such an event the engineered Glutamine Synthetase activity is useful in cell survival and for driving recombinant gene expression.

Figure 1: Glutamine Synthetase Technology Principle
Advantages of GenScript CHO Glutamine Synthetase Technology
While there are several advantages to the use of CHO Glutamine Synthetase technology, the 3 most important attributes would be Productivity, Speed and Stability.
Figure 2: Productivity, Speed and Stability are 3 important advantages when using GenScript CHO Glutamine Synthetase Technology
GenScript CHO Glutamine Synthetase Technology Productivity
Use of GenScript Glutamine Synthetase technology will ensure the establishment of economic processes. GenScript CHO Glutamine Synthetase technology reliably results in high yielding cell lines. While the maximum expression levels attainable depend on the product, GenScript has created cell lines producing >5 g/L rAb with production rates in the range of 50-100 pcd.
-
Figure 3: Maximizing volumetric productivity is essential for lowering cost, especially in the manufacturing runs.
-
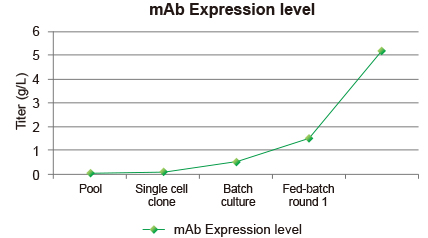
Figure 4: Going from pool to round 1 fed batch, GenScript can achieve >g/L expression. Going into round 2 fed batch can take it beyond 5 g/L.
GenScript CHO Glutamine Synthetase Technology Speed
Cell line development speed is crucial to overall project success. GenScript CHO-Glutamine Synthetase technology allows for rapid selection of high yielding cell lines during the first screen. Even higher yields are achieved through media and process optimization. This significantly reduces the time required to generate a cell line suitable for GMP process. Also, since the GenScript CHO-Glutamine Synthetase cell line is pre-adapted to growth in chemically defined, animal free media and suspension culture, it aids adaptation of the cell line back to growth in suspension culture after transfection.
| Milestone | Weeks | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| Pool | |||||||||||||||||
| Single Cell Clone | |||||||||||||||||
| Batch Culture | |||||||||||||||||
| Fed-Batch Culture | |||||||||||||||||
Figure 5: Gram-level mAb productivity is achievable in 14-17 weeks
GenScript CHO Glutamine Synthetase Technology Stability
CHO Glutamine Synthetase cell lines that are stable in terms of product concentration, growth and product characteristics are created using GenScript CHO Glutamine Synthetase technology.
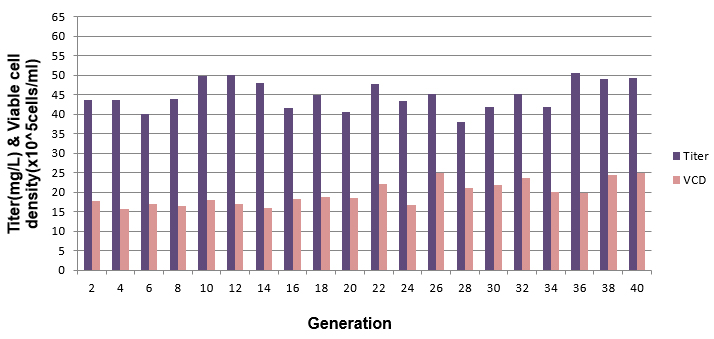
Figure 6: Cell lines maintained over a period of 40 generations demonstrated consistent product titer and viable cell densities.
GenScript CHO Glutamine Synthetase Technology Summary
The ultimate goal of cell line development is to obtain clonal cell lines that produce the desired protein or recombinant antibody (rAb) with high specific productivity, at consistently high levels, over an extended number of generations, allowing for scalable and cost-effective processes. GenScript CHO Glutamine Synthetase Technology can help realize this goal. For details on the GenScript CHO Glutamine Synthetase service click here.
For details on GenScript's overall stable cell line service portfolio, click here.
Not what you are looking for? See our related service pages below.
- Prefer to check on your protein expression level first? Check out our PROTential protein expression evaluation & optimization service. PROTential helps to check on the target protein expression in CHO or HEK293 cells, starting at just $500.
- Looking for antibody fragment production services? Visit our guaranteed antibody fragments production page.
- Want up to 1 mg purified rAb for antibody drug screening applications, in a High Throughput (HTP) format? Visit our HTP Gene to Antibody page.
- Need high quality recombinant antibody up to 500 mg at ≥95% purity and with low endotoxin levels? Visit our MamPower™ guaranteed recombinant antibody production page.
- Need more than 500 mg of recombinant antibody? Visit our large scale recombinant antibody production page.
- Need high quality mammalian protein expression services? Visit our MamPower™ guaranteed protein expression page.
- For information about our protein services, please visit our recombinant protein services main page or contact us using the information listed below.
- Unsure which rAb service would work best for you? Use this guide to quickly determine which services are the best match for your application.

Our customer service representatives are available 24 hours a day, Monday through Friday to assist you.
