-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- Gene Synthesis FLASH Gene
- GenBrick™ Up to 200kb
- Gene Fragments Up to 3kb now
- Plasmid DNA Preparation Upgraded
- Cloning and Subcloning
- ORF cDNA Clones
- mRNA Plasmid Solutions New!
- Cell free mRNA Template New!
- AAV Plasmid Solutions New!
- Mutagenesis
- GenCircle™ Double-Stranded DNA New!
- GenSmart™ Online Tools
-
-
PRODUCTS
-
Most Popular Reagents
-
 Instruments
Instruments
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Resources
-
- All Instruments
- Automated Protein and Antibody Purification SystemNew!
- Automated Plasmid MaxiprepHot!
- Automated Plasmid/Protein/Antibody Mini-scale Purification
- eBlot™ Protein Transfer System
- eStain™ Protein Staining System
- eZwest™ Lite Automated Western Blotting Device
- CytoSinct™ 1000 Cell Isolation Instrument
-
- Pharmacokinetics and Immunogenicity ELISA Kits
- Viral Titration QC ELISA Kits
- -- Lentivirus Titer p24 ELISA KitHot!
- -- MuLV Titer p30 ELISA KitNew!
- -- AAV2 and AAVX Titer Capsid ELISA Kits
- Residual Detection ELISA Kits
- -- T7 RNA Polymerase ELISA KitNew!
- -- BSA ELISA Kit, 2G
- -- Cas9 ELISA KitHot!
- -- Protein A ELISA KitHot!
- -- His tagged protein detection & purification
- dsRNA ELISA Kit
- Endonuclease ELISA Kit
- COVID-19 Detection cPass™ Technology Kits
-
- Automated Maxi-Plasmid PurificationHot!
- Automated Mini-Plasmid PurificationNew!
- PCR Reagents
- S.marcescens Nuclease Benz-Neburase™
- DNA Assembly GenBuilder™
- Cas9 / Cas12a / Cas13a Nucleases
- Base and Prime Editing Nucleases
- GMP Cas9 Nucleases
- CRISPR sgRNA Synthesis
- HDR Knock-in Template
- CRISPR Gene Editing Kits and Antibodies
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![ELISA Kits]() ELISA Kits
ELISA Kits
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Stable Cell Lines]() Stable Cell Lines
Stable Cell Lines
-
![Cell Isolation and Activation]() Cell Isolation and Activation
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
Resources » Learning Center » Research Digest » New Approach Leverages Antibodies to Acid-Base Transporters in Breast Cancer
New Approach Leverages Antibodies to Acid-Base Transporters in Breast Cancer
Summary
Acidification of the tumor microenvironment is generally accepted as a factor that promotes carcinogenesis processes. Therefore, understanding the relationship between pH dynamics and cancer progression is relevant to developing new therapeutic strategies. Recent studies highlighted a connection between the overexpression of the acid-base transporter NBCn1 (SLC4A7) and breast cancer malignancy. Here, a team at Aarhus University, Denmark, led by Ebbe Boedtkjer, has developed inhibitory anti-NBCn1 antibodies to directly dissect the role of this molecule in acid extrusion in breast cancer, providing support for a new potentially effective strategy to target metastatic disease.
Background
Tumors are surrounded by a complex microenvironment, including stromal tissue, infiltrating immune cells, soluble secreted factors, extracellular matrix, and more. The tumor’s activities influence the properties of this complex and dynamic microenvironment. Concurrently, it’s well known that the microenvironment critically modulates several tumor cell behaviors, such as proliferation, migration, and metastasis.1 For instance, the combination of cancer cells’ high metabolic rate and poor circulation at the tumor site results in interstitial acidification, creating a permissive microenvironment for tumor growth.2
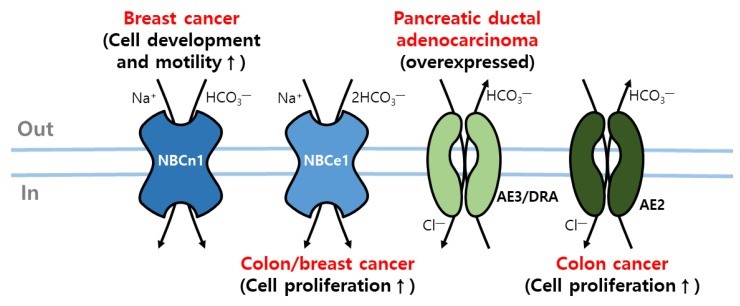
“Upregulated bicarbonate transporters in cancer cells. The activation of NBCn1 and the expression of CBE (AE3 and DRA) is upregulated in breast cancer and pancreatic ductal adenocarcinoma. In addition, NBCe1 and AE2 activation increases colon cancer cell proliferation.” Retrieved without modifications from Lee and Hong, 2020.2 https://creativecommons.org/licenses/by/4.0/
Additionally, bicarbonate transporters critical for cellular pH regulation are often overexpressed in various tumor types, increasing intracellular pH and interstitial acidification and influencing carcinogenesis processes.2,3
How changes in pH homeostasis modulate cancer cell behaviors is not fully elucidated. However, in vitro evidence is mounting, connecting the higher intracellular pH in cancer cells with their higher metabolism and increased DNA and protein synthesis. Overall, overexpression of acid-base transporters is known to boost cancer cell proliferation, motility, and invasion.3
Among various pH-regulating transporters implicated in the carcinogenesis process, a recent study links increased expression of the sodium/bicarbonate cotransporter NBCn1 (SLC4A7) with higher malignancy-grade breast cancer and reduced survival.3 Thereby, NBCn1 has emerged as a relevant target for cancer therapy.
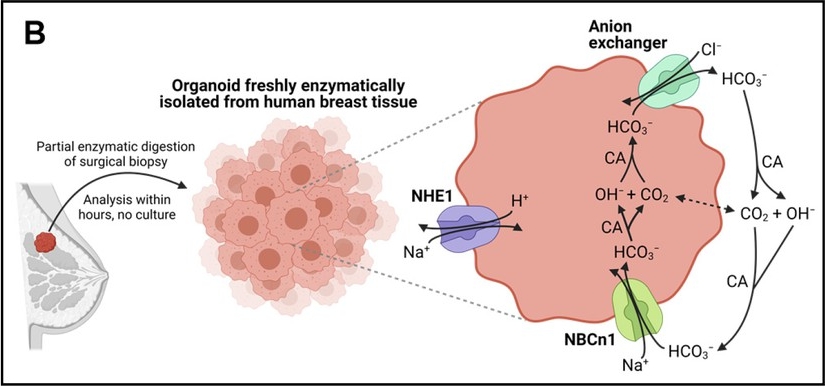
“Cellular net acid extrusion in human breast cancer tissue and normal breast tissue relies on extracellular Na+ and is partially CO2/HCO3–-dependent consistent with the expression of Na+, HCO3– cotransporter NBCn1 and Na+/H+ exchanger NHE1. Moreover, steady-state intracellular pH (pHi) and the capacity for net acid extrusion are elevated in human breast cancer tissue compared to normal breast tissue.” Retrieved from Toft et al. 2021, only Figure 1, panel B is shown.3 https://creativecommons.org/licenses/by/4.0/
To better understand the role of NBCn1 in breast cancer progression, a team at Aarhus University, Denmark, led by Ebbe Boedtkjer, has developed anti-NBCn1 antibodies targeting the cotransporter’s extracellular epitopes.4 By developing antibodies to inhibit NBCn1 specifically, the team was able to probe the role of this sodium/bicarbonate cotransporter in pH regulation and its involvement in growth and metastasis in human breast cancer. Significantly, this work by Axelsen and colleagues provides support for developing anti-NBCn1-based therapeutic strategies for breast cancer.4
Developing a functional monoclonal antibody against NBCn1
To develop a monoclonal antibody targeting extracellular epitopes of the NBCn1 sodium/bicarbonate cotransporter, Axelsen and colleagues selected a peptide sequence within the third extracellular loop having low homology to other closely related SLC4 family proteins. Peptide immunogen synthesis, mouse immunization, and generation of monoclonal antibodies through hybridoma technology were outsourced to GenScript. Through this partnership, the team successfully identified various antibody candidates with the correct specificity and a high-affinity binder (5H2.1, KD = 0,14 nM). The team also secured the lead’s variable domain sequence for cloning and recombinant antibody production.
The 5H2.1 recombinant antibody showed specificity, only binding to the immunogen peptide corresponding to the NBCn1 human sequence and not binding to either the corresponding mouse peptide or that from other SLC4 transportes, such as NBCe1. Significantly, they found that their lead recombinant antibody was functional, efficiently inhibiting the transport of sodium/bicarbonate in cells overexpressing NBCn1.
Validating the role of NBCn1 in breast cancer
With this new powerful tool, Axelsen and colleagues could finally interrogate the acid extrusion role of NBCn1 in human breast cancer tissue. Such evaluation was previously challenging due to the unspecificity of available inhibitory small molecules.
Leveraging organoids developed from fresh biopsied breast cancer tissue, the team demonstrated an absolute inhibition of sodium/bicarbonate transport by the anti-NBCn1 5H2.1 monoclonal antibody. Next, they aimed to validate a previous finding correlating NBCn1 expression with breast cancer severity. By evaluating organoids from patients with different levels of metastatic disease, the team showed a correlation between antibody-mediated acid extrusion inhibition and disease severity. This finding supports the value of NBCn1 expression as a predictive marker for regional lymph node metastasis in human primary breast carcinomas.5
Previous preclinical work had also connected NBCn1 overexpression with breast tumor growth. Hence, Axelsen and colleagues leveraged the anti-NBCn1 5H2.1 monoclonal antibody in breast tumor xenograft mouse models to validate this effect. For model development, the team selected triple-negative breast cancer patient-derived tumor tissues with extensive NBCn1 expression. Reduced tumor growth was significant in one of the two models tested, and the anti-NBCn1 antibody did not induce any adverse effects in mice. Overall, the team found that reduced tumor growth was attributable to cell cycle arrest and apoptosis effects of anti-NBCn1 antibody treatment.
Conclusion
In this study, Axelsen and colleagues developed tools to probe the role of acidification processes in breast carcinogenesis. Their previous findings correlated the activity and expression of acid-base transporters, such as NBCn1, with breast cancer proliferation and invasiveness.3 However, to fully understand the dynamics at play, the team was missing the means to specifically target and inhibit the NBCn1 cotransporter. Careful selection of the extracellular NBCn1 sequence to serve as an immunogen enabled the generation and discovery of monoclonal antibodies with desirable selectivity, specificity, and function. By leveraging these inhibitory anti-NBCn1 antibodies in various in vitro and in vivo models of highly malignant breast cancer, Axelsen and colleagues have demonstrated the relevance of this specific sodium/bicarbonate cotransporter for acid extrusion and its critical role in carcinogenesis. Importantly, these studies may open the door to a novel therapeutic approach against metastatic breast tumors for improved survival.
References
1. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020 Aug 17;30(16):R921-R925. https://doi.org/10.1016%2Fj.cub.2020.06.081.
2. Lee D, Hong JH. The Fundamental Role of Bicarbonate Transporters and Associated Carbonic Anhydrase Enzymes in Maintaining Ion and pH Homeostasis in Non-Secretory Organs. Int J Mol Sci. 2020 Jan 4;21(1):339. doi: 10.3390/ijms21010339.
3. Nicolai J ToftTrine V AxelsenHelene L PedersenMarco MeleMark BurtonEva BallingTonje JohansenMads ThomassenPeer M ChristiansenEbbe Boedtkjer (2021) Acid-base transporters and pH dynamics in human breast carcinomas predict proliferative activity, metastasis, and survival eLife 10:e68447. https://doi.org/10.7554/eLife.68447.
4. Axelsen, T.V., Olesen, C., Khan, D. et al. Antibodies toward Na+,HCO3–-cotransporter NBCn1/SLC4A7 block net acid extrusion and cause pH-dependent growth inhibition and apoptosis in breast cancer. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02591-0.
5. Toft NJ, Axelsen TV, Pedersen HL, Mele M, Burton M, Balling E, Johansen T, Thomassen M, Christiansen PM, Boedtkjer E. Acid-base transporters and pH dynamics in human breast carcinomas predict proliferative activity, metastasis, and survival. Elife. 2021 Jul 5;10:e68447. https://doi.org/10.7554/elife.68447.

-





































